Abstract
Background:
Mucoepidermoid carcinoma (MEC) shows differences in biological behaviour depending mainly on its histological grade. High-grade tumours usually have an aggressive biological course and they require additional oncological treatment after surgery.
Methods:
In a series of 43 MECs of the salivary glands, we studied the epidermal growth factor receptor (EGFR) gene by using dual-colour chromogenic in situ hybridisation (CISH). Moreover, we assessed the protein expressions of the EGFR and the activated extracellular signal-regulated kinases (pERK1/2) by using immunohistochemistry. These results were correlated with the histological grade of the tumours and the outcome of the patients.
Results:
The CISH study demonstrated a high-EGFR gene copy number, with balanced chromosome 7 polysomy, in 8 out of 11 high-grade MECs (72.7%), whereas 27 low-grade and 15 intermediate-grade tumours had a normal EGFR gene copy number (P<0.001). The EGFR gene gains correlated with disease-free interval (P=0.003) and overall survival of the patients (P=0.019). The EGFR protein expression had a significant correlation with the histological grade of the tumours but not with the outcome of the patients. The pERK1/2 expression correlated with histological grade of tumours (P<0.001), disease-free interval (P=0.004) and overall survival (P=0.001).
Conclusions:
The EGFR/ERK pathway is activated in high-grade MECs with aggressive behaviour. Patients with these tumours who require oncological treatment in addition to surgery could benefit from EGFR and mitogen-activated protein kinase pathway inhibitors.
Similar content being viewed by others
Main
Mucoepidermoid carcinoma (MEC) is the most frequent malignant tumour that originates in the major and minor salivary glands, and represents about one third of all malignant salivary gland tumours (Spiro, 1986; Goode and El-Naggar, 2005; Ellis and Auclair, 2008). It is a heterogeneous neoplasm that may present different biological behaviour, depending mainly on the histological grade of the tumour (Goode et al, 1998; Goode and El-Naggar, 2005; Ellis and Auclair, 2008; Nance et al, 2008). Surgical resection is the standard treatment for all grades of MEC. Radiotherapy after wide surgical excision of the tumour is recommended for high-grade MECs. Lymphadenectomy and adjunvant external beam radiotherapy are indicated when cervical metastases are present. Chemotherapy is indicated in the treatment of metastatic disease and in the palliation of locoregional disease not amenable to either salvage surgery or radiation therapy (Agulnik and Siu, 2004; Nance et al, 2008). Low-grade MECs usually do not recur; most patients are cured after surgery and the 5-year survival rate is 76–95%. Conversely, high-grade MECs are aggressive neoplasms that frequently have an infiltrative pattern of growth, recur and even metastasize, and their 5-year survival rate is 30–50% (Goode et al, 1998; Nance et al, 2008).
To date, clinical trials using targeted therapies on salivary gland tumours are scarce, probably because of the low number of these cases in each institution. Only one phase II study of Herceptin (Trastuzumab) with disappointing results in patients with advanced salivary gland tumours overexpressing HER2/neu has been published (Haddad et al, 2003). Incomplete clinical trials using epidermal growth factor receptor (EGFR) antagonists have been performed on patients with salivary gland cancer. Moreover, studies on the oncogenetic pathways in salivary gland MECs predictive of response to targeted therapies are scarce and incomplete. In recent years, strategies against the EGFR family and the mitogen-activated protein kinase (MAPK) signalling pathway have received special attention in the treatment of cancer. The EGFR family, including the four distinct receptors EGFR/ErbB1, HER2/cerbB2, HER3/CerbB3 and HER4/ErbB4, has been identified as a potential therapeutic target in solid tumours. The EGFR/ErbB1 is a gene located on chromosome 7p12 and has emerged as a significant factor in the development and growth of many types of cancer, playing an important role in cancer-cell proliferation, angiogenesis and metastasis. This gene encodes a 170-kDa membrane glycoprotein that can be activated by phosphorylation and induce a downstream signalling transduction cascade. A major signalling route of the EGFR is the Ras-Raf-MAPK pathway (Klapper et al, 2000). Activation of Ras initiates a multistep phosphorylation cascade that leads to the activation of MAPKs. The MAPK extracellular signal-regulated kinases ERK1/2 are the best characterised and are most strongly associated with human cancer. The ERK1/2 are activated by dual phosphorylation on a tyrosine and a threonine residue by dual-specificity kinases, and subsequently regulate cell transcription and have been linked to proliferation, survival and transformation (Lewis et al, 1998).
The EGFR antagonists are included in treatment protocols of advanced stages of non-small cell carcinoma of the lung, colorectal cancer and head and neck squamous cell carcinoma (Ciardello and Tortora, 2008). In head and neck tumours, EGFR can be abnormally activated and protein overexpression by the neoplastic cells is frequently detected by immunohistochemistry (Nicholson et al, 2001; Grandis and Sok, 2004). However, EGFR amplifications are not frequent and EGFR activating mutations are very rare (Kalyankrishna and Grandis, 2006). The EGFR overexpression has been correlated with poor prognosis in head and neck cancer (Kalyankrishna and Grandis, 2006). To date, several clinical trials have been carried out to identify the molecular characteristics of the tumours predictive of response to EGFR antagonists. The EGFR activating mutations and increased EGFR gene copy number identify the most sensitive population in these tumours (Cappuzzo et al, 2005; Hirsch et al, 2005; Tsao et al, 2005; Sartore-Bianchi et al, 2007). In most MECs of the salivary gland, the EGFR protein is overexpressed (Gibbons et al, 2001; Shang et al, 2007), but EGFR activating mutations are extremely rare (Han et al, 2008; Dahse and Kosmehl, 2008; Dahse et al, 2009). However, studies on the EGFR gene copy number have not been performed before in a series of salivary gland MECs.
In our previous studies, we saw that high-grade MECs with aggressive course differ molecularly from low-grade tumours. These high-grade tumours overexpress the oncogenic glycoprotein MUC1 (Alos et al, 2005; Handra-Luca et al, 2005). MUC1 acts as a proto-oncogene that interacts with EGFR and correlates with MAPK activation in mouse models (Schroeder et al, 2001) and inhibits the ligand-mediated ubiquitinisation and degradation of EGFR in vitro (Pochampalli et al, 2007). Moreover, the expression of ERK1/2 MAPKs has been related to aggressive tumour behaviour in MECs of the salivary glands (Handra-Luca et al, 2003). We therefore hypothesised that histological high-grade MECs, which have a clinically aggressive course, may harbour EGFR protein overexpression and high-EGFR gene copies linked to aggressive tumour biology. To investigate this, we studied the EGFR gene by using chromogenic in situ hybridisation (CISH) with a dual-colour probe, in a series of 43 MECs. This new technique obtains the same results as fluorescence in situ hybridisation (FISH) and offers potential advantages over FISH to detect gene copy number, including the ability to distinguish between areas of tumour and normal tissue.
In addition to genetic analysis, the immunohistochemical study of the EGFR protein was performed and activated ERK1/2 were assessed by using an antibody specific for the dually phosphorylated and activated ERK1 and ERK2 (MAPK phospho-p44/42). These molecular studies have been correlated with the histological characteristics of the tumours and the follow-up of the patients.
Materials and methods
Selection of cases
Forty-three MECs diagnosed at the Department of Pathology of the Hospital Clinic, and Hospital Princeps d’Espanya, Bellvitge, University of Barcelona, from 1996 until 2005, were reviewed. The medical records were obtained from patients’ files in the Departments of Otorhinolaryngology and Maxillofacial Surgery. The study was approved by the Local Ethical Committee and patients gave their informed consent. At diagnosis, the tumours were staged according to the American Joint Committee on Cancer (Sobin and Wittekind, 2002). All patients underwent primary surgery as standard treatment. Lymph node dissection was performed only in cases with lymph node metastases. Full-dose radiotherapy was applied after tumour excision with positive margins, when lymph node metastases were assessed, and in locoregional recurrences. Chemotherapy with cisplatin was added for palliative purposes, in patients with lymph node metastases (N2 or N3) and in cases with tumoural persistence after surgery and resistance to radiotherapy.
Histological grading of MECs
Haematoxylin-eosin and alcian blue-stained slides and paraffin wax-embedded material were available for all cases. The MECs were graded following the 2005 World Health Organization Classification of Tumours (Goode and El-Naggar, 2005).
CISH and immunohistochemistry
Representative paraffin wax blocks were selected from each of the 43 cases for CISH and immunohistochemistry.
The CISH was performed on a 4-μm section of each tumour that was deparaffined in two changes of xylene and three washes of degraded ethanol for 3 min each. The slides were pretreated with CISH pretreatment buffer (Dako, Carpinteria, CA, USA) and heated to 92°C, and then rinsed with distilled water. The tissues were digested for 10 min with pepsin digestion solution (Dako) at room temperature, washed twice in distilled water for 5 min each, dehydrated in 70, 85 and 96% alcohol for 2 min each and dried. A measure of 10 μl of dual-colour EGFR Spectrum-red/CEP7 Spectrum-blue probe (Dako) were applied to each slide. Sections were covered with coverslips and denatured on a hot plate at 82°C for 5 min. Hybridisation was done overnight at 37°C. Then the slides were washed in 2 × SSC at 73°C for 2 min and three times in distilled water. Then the sections were blocked with H2O2 in absolute methanol and incubated with a blocking reagent for 10 min at room temperature. The hybridisation signals were detected after sequential incubations with anti-mouse anti-DIG (60 min at room temperature), polymerised horseradish peroxidase anti-mouse antibody (60 min) and 3,3-diaminobenzedine (DAB). The sections were counterstained with haematoxylin.
Immunohistochemical studies were carried out using the automated immunohistochemical system TechMate 500 (Dako), and the EnVision system (Dako). Briefly, 4 μm sections were deparaffinised and hydrated using graded alcohols and water. For antigen retrieval, an autoclave pretreatment at 120°C for 5 min was performed. Peroxidase was blocked for 7.5 min in ChemMate peroxidase-blocking solution (Dako). The slides were incubated with the primary antibodies for 30 min and washed in ChemMate buffer solution (Dako). The peroxidase labelled polymer was then applied for 30 min. After being washed in ChemMate buffer solution, the slides were incubated with DAB substrate chromogen solution, washed in water, counterstained with haematoxylin, washed, dehydrated and mounted. The primary antibodies used in the study were: EGFR (Dako; dilution 1 : 100) and pERK1/2 (Phospho-p44/42; Thr202/Tyr204) (Cell Signaling Technology, Beverly, MA, USA; dilution 1 : 50). Appropriate positive and negative controls were used.
The CISH and immunohistochemical results were evaluated by two independent observers (BL and LA). For CISH evaluation, a light microscope under a × 40 objective was used. A total number of 100 tumoural cells were evaluated. The centromeric blue signal and the EGFR red signal in each cell were counted and the proportion centromeric/EGFR signal number was calculated. The cases were considered normal if two blue and two red signals were visualised in each nuclear cell. Polysomy was considered when ⩾3 blue and red signals (in equal number) were seen in each nucleus. The EGFR amplification was defined as red signals >1.5 blue signals.
The immunostain for EGFR was evaluated: 0, no positive cells; 1+, low discontinuous membrane staining; 2+, unequivocal membrane staining with moderate intensity and 3+, strong and complete membrane staining. Only cases with 2+ and 3+ staining patterns were considered positive. The pERK1/2 showed nuclear positivity. For analytical purposes, positivity for EGFR and pERK1/2 was considered when ⩾10% of tumour cells were positive. High-pERK1/2 expression was considered when ⩾30% of positive cells were detected.
Statistical analysis
The continuous clinical variables considered were follow-up and age (median and range were calculated). Overall survival was calculated from diagnosis to the death of the patient or loss of follow-up. Disease-free interval was the time from surgical excision of the tumour to the first recurrence or metastasis. Both overall survival and disease-free interval were analysed by the Kaplan–Meier method. The categorical clinical variables were gender (female/male), location of tumours (parotid/submaxillary/minor salivary gland) and stage (I/II/III/IV). The categorical histological variables considered were histological grade (1/2/3) and molecular results: EGFR protein expression (positive/negative), EGFR gene copy (normal/polysomy) and ERK1/2 expression (>30% of positive cells/<30% of positive cells). Fisher's exact test was used for comparison between qualitative variables and Student's t-test and ANOVA were applied for quantitative variables according to the application conditions. All tests were two sided. Differences were analysed by the log-rank method. Differences were considered to be statistically significant with an α risk of 0.05.
Results
Clinicopathological characteristics of the patients
The clinicopathological characteristics of the patients at diagnosis, the treatment details and outcome are summarised in Table 1.
After a median follow-up of 62 months, 33 (76.8%), 6 (13.9%) and 4 (9.3%) patients were alive and disease free, alive with disease and died of disease, respectively. The median disease-free interval was 96 months (range 0–159 months). Relapses occurred in 19 (44.1%) patients: in 14 (32.6%) patients, a local tumoural recurrence took place, and in 5 (11.6%) patients, there was lymph node metastasis.
The statistical associations of the disease-free interval and overall survival with histological grade of tumours and molecular results are expressed in Table 2. Patients with high-grade tumours had shorter disease-free interval (P=0.001) and overall survival (P=0.001) than those with low- and intermediate-grade tumours.
EGFR gene analysis
Eight cases (18.6%) had chromosome 7 polysomy. In these cases, there were >2 signals of both centromere and EGFR signals in over 70% of cells, but the relationship between both signals was 1 : 1. In two cases there were 3 signals (low polysomy) and in six cases there were >3 signals (high polysomy). No cases with EGFR amplification were detected. All of the eight cases with chromosome 7 polysomy were high-grade MECs, whereas the rest of the tumours (27 low grade, 5 intermediate grade and 3 high grade) showed a normal pattern of expression (P<0.001). Chromosome 7 polysomy was associated with shorter disease-free interval (P=0.003) and overall survival (P=0.019) (Figure 1).
EGFR and pERK1/2 protein expression
The EGFR protein expression was positive in 34 tumours (79%). All cases with chromosome 7 polysomy showed expression of the EGFR protein (P<0.001). These cases showed positivity in >60% of tumoural cells. High-EGFR protein expression was associated with high-histological grade of tumour (P<0.001, ANOVA), but it was associated with neither disease-free interval (P=0.286) nor overall survival (P=0.307).
The pERK1/2 protein was expressed in 34 tumours (79%). There was a statistical correlation between pERK1/2 positivity and histological grade of tumour (P<0.001, ANOVA), shorter disease-free interval (P=0.004) and overall survival (P=0.001). High-pERK1/2 expression (positivity in ⩾30% of neoplastic cells) was observed in 21 tumours (49%). High-pERK1/2 expression was associated with shorter overall survival (P=0.025) (Figure 2), but not with disease-free interval (P=0.108). All cases with EGFR polysomy had high expression of pERK1/2 (P=0.002) and there was a marginally significant correlation between high expression of pERK1/2 and EGFR immunohistochemical expression (P=0.047) (Figure 3).
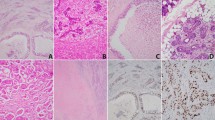
An example of high-grade mucoepidermoid carcinoma. (A) Histological characteristics of the neoplasm (HE × 200). (B) The CISH analysis shows high polysomy. Four or five signals (both red EGFR and blue centromere) are seen in each nucleus in most of the neoplastic cells (EGFR CISH × 630). (C) Expression of EGFR protein with strong and diffuse membrane positivity (EGFR × 400). (D) High expression of activated ERK1/2 with nuclear positivity in most of the neoplastic cells (pERK1/2 × 400).
Discussion
This study shows that high-grade MECs with aggressive behaviour harbour an increased EGFR gene copy number and high expression of pERK1/2 MAPKs. In spite of the fact that EGFR amplification was not seen in any of the 43 cases of this series, in six of them there was high polysomy with ⩾4 EGFR gene copies. The EGFR gene is rarely amplified in human cancers, but the increased EGFR gene copy number with balanced chromosome 7 polysomy in cancer cells is relatively frequent, in ∼24–40% of patients with non-small cell lung cancer, squamous-cell carcinoma of the head and neck or colorectal cancer. Chromosome 7 polysomy has been linked to tumour aggressiveness and poor clinical outcome (Hirsch et al, 2003; Ciardello and Tortora, 2008). In this study, all cases with EGFR gene gains had a significant shorter disease-free interval and overall survival. The EGFR product is a membrane glycoprotein composed of an extracellular ligand-binding domain, a transmembrane lipophilic component and an intracellular protein kinase domain. The ligand binding induces EGFR dimerisation, activation of the intrinsic tyrosine kinase protein and tyrosine phosphorylation with the activation of a cascade of biochemical and physiological responses (Lewis et al, 1998). This downstream signalling transduction activates MAPKs through phosphorylation by MAPK kinases, and the activation of this pathway is associated with cell proliferation and oncogenic transformation (Grandis and Sok, 2004). In this series, there was a significant correlation between increased EGFR copy number and high expression of pERK1/2 (P=0.002). High expression of activated ERK1/2 has been related to tumour progression in several neoplasms (Albanell et al, 2001; Adeyinka et al, 2002) and in salivary gland MECs (Handra-Luca et al, 2003). In this series, the pERK1/2 expression was significantly correlated with shorter disease-free interval and overall survival. Furthermore, MAPKs can also be activated through the upstream activation of HER2/neu or RAS. About one third of salivary gland MECs have HER2/neu gene amplification (Press et al, 1994) and about one fifth of MECs harbour H-RAS mutations (Yoo and Robinson, 2000a), but K-RAS mutations are extremely rare (Yoo and Robinson, 2000b). However, to define the molecular mechanisms underlying the biological behaviour in high-grade MECs, in vitro experiments with cell lines should be carried out.
The immunohistochemical expression of EGFR in the majority of MECs that we have observed is concordant with other studies (Gibbons et al, 2001; Shang et al, 2007). All cases with chromosome 7 polysomy had an expression of EGFR protein of over 60% of cells. Nevertheless, most immunohistochemical positive cases failed to show an increased EGFR gene copy number. This discrepancy between the EGFR gene copy number and the immunohistochemical detection of the protein has been reported before in several cancers, and has been attributed to a post-transcriptional phenomenon mediated at the mRNA level (Grandis and Tweardy, 1993). There was a significant correlation between the EGFR protein expression and the histological grade of the tumours, but not with the clinical outcome of the patients. The current grading system classification is three-tiered, and tumours are classified into low-, intermediate- and high-grade MECs, depending on the architecture and cellular characteristics of the neoplasms. Low-grade tumours are usually well-defined tumours, often cystic with a predominance of mucous cells, whereas high-grade MECs usually have an infiltrative pattern of growth, are solid and mainly composed of intermediate type and epidermoid cells. High mitotic index, cellular anaplasia, necrosis and perineural invasion are characteristics of high-grade tumours (Spiro, 1986; Ellis and Auclair, 2008). Significant differences in the outcome of the patients related to histological grade have been repeatedly confirmed in series of MECs of the salivary glands (Goode et al, 1998; Alos et al, 2005; Nance et al, 2008). In this study, a statistical correlation between the histological grade and disease-free interval and overall survival of the patients was found. The prognostic value of the EGFR polysomy, and the EGFR and the pERK1/2 protein expressions were related to the histological grade.
Strategies against EGFR include monoclonal antibodies able to bind to the extracellular domain of the receptor such as cetuximab, or small molecule ATP-competitive tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib. Some clinical, histopathological and molecular characteristics have been proposed for identifying the population sensitive to EGFR-TKI treatment in non-small carcinoma of the lung (Sone et al, 2007). Activating mutation in exons 18, 19 and 21 of the EGFR gene has proved to be a significant factor in predicting response to EGFR-TKIs in non-small cell carcinoma of the lung. However, these mutations are less common in the United States and European population than in the Asian population (Sone et al, 2007), and data from large randomised studies indicate that increased EGFR gene copy number is probably the best factor in predicting response and evaluate overall survival of the patients (Hirsch et al, 2005; Tsao et al, 2005; Cappuzzo et al, 2005). Interestingly, a good response to EGFR antagonists in head and neck and lung carcinomas with expression of MAPKs has been observed (Albanell et al, 2001; Gandara et al, 2004). Moreover, immunohistochemical positivity for activated ERK1/2 has been correlated with a good response to MAPKs inhibitors in clinical trials on cutaneous melanomas (Jilaveanu et al, 2009). Therefore, the high-grade MECs in this series, with increased EGFR gene copy number and pERK1/2 high expression could be sensitive to EGFR or MAPKs antagonists.
The MECs of the lung share histological and molecular characteristics with salivary gland MECs. Some series on lung MECs have shown lack of EGFR mutations in these tumours and a percentage of chromosome 7 polysomy of 17%, similar to the results in our series (Macarenco et al, 2008). However, some lung MECs have been described as having activating EGFR mutations in the Asian population (Han et al, 2008). The MECs and adenosquamous carcinomas share histological characteristics and differential diagnosis between both tumour types may be challenging in the head and neck region and lung (Alos et al, 2004; Rossi et al, 2009). Adenosquamous carcinomas are aggressive tumours arising from upper or lower airways, whereas MECs have a salivary or bronchial gland origin, whose prognosis depends on the histological grade. Adenosquamous carcinomas usually harbour EGFR activating mutations, whereas MECs do not (Kang et al, 2007; Han et al, 2008; Macarenco et al, 2008; Rossi et al, 2009). Previous studies on salivary gland MECs have found that EGFR mutations are extremely rare (Han et al, 2008; Dahse and Kosmehl, 2008; Dahse et al, 2009).
To date, few cases on metastatic salivary gland MECs with EGFR gene gains with chromsome 7 polysomy and good response to EGFR monoclonal antibody cetuximab have been published (Grisanti et al, 2008). However, clinical trials that include a large series of salivary gland MECs are difficult to carry out because of the low number of these cases in each institution.
In conclusion, we have identified that high-grade salivary gland MECs usually have an increased EGFR gene copy number and highly express pERK1/2. The activation of the EGFR/ERK pathway in these tumours is associated with aggressive behaviour and could represent potential indicators of response to EGFR antagonists or MAPK pathway inhibitors.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC (2002) Activated mitogen-activated protein kinase expression during human breast tumourigenesis and breast cancer progression. Clin Cancer Res 8: 1747–1753
Agulnik M, Siu LL (2004) An update on the systemic therapy of malignant salivary gland cancers: role of chemotherapy and molecular targeted agents. Curr Med Chem 4: 543–551
Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Rosello J, Nicholson RI, Mendelsohn J, Baselga J (2001) Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res 61: 6500–6510
Alos L, Castillo M, Nadal A, Caballero M, Mallofre C, Palacin A, Cardesa A (2004) Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology 44: 570–579
Alos L, Lujan B, Castillo M, Nadal A, Carreras M, Caballero M, de Bolos C, Cardesa A (2005) Expression of membrane-bound mucins (MUC1 and MUC4) and secreted mucins (MUC2, MUC5AC, MUC5B, MUC6 and MUC7) in mucoepidermoid carcinomas of salivary glands. Am J Surg Pathol 29: 806–813
Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I, Ludovini V, Magrini E, Gregorc V, Doglioni C, Sidoni A, Tonato M, Franklin WA, Crino L, Bunn PA, Varella-Garcia M (2005) Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non–small-cell lung cancer. J Natl Cancer Inst 97: 643–655
Ciardello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358: 1160–1174
Dahse R, Driemel O, Schwartz S, Dahse J, Kromeyer-Hauschild K, Berndt A, Kosmehl H (2009) Epidermal growth factor receptor kinase domain mutations are rare in salivary gland carcinomas. Br J Cancer 100: 623–625
Dahse R, Kosmehl H (2008) Detection of drug-sensitizing EGFR exon 19 deletion mutations in salivary gland carcinoma. Br J Cancer 99: 90–92
Ellis GL, Auclair PL (2008) Mucoepidermoid carcinoma. In Atlas of Tumour Pathology. Tumours of the Salivary Glands, Silverberg SG, Sobin LH (eds), pp 173–193. Armed Forces Institute of Pathology: Washington, DC
Gandara DR, West H, Chansky K, Davies AM, Lau DHM, Crowley J, Gumerlock PH, Hirsch FR, Franklin WA (2004) Bronchioloalveolar carcinoma: a model for investigating the biology of epidermal growth factor receptor inhibition. Clin Cancer Res 10: 4205s–4209s
Gibbons MD, Manne U, Carroll WR, Peters GE, Weiss HL, Grizzle WE (2001) Molecular differences in mucoepidermoid carcinoma and adenoid cystic carcinoma of the major salivary glands. Laryngoscope 111: 1373–1378
Goode RK, Auclair PL, Ellis GL (1998) Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 82: 1217–1224
Goode RK, El-Naggar AK (2005) Mucoepidermoid carcinoma. In World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours, Barnes L, Eveson JW, Reichart P, Sidransky D (eds), pp 219–220. IARC Press: Lyon
Grandis JR, Sok JC (2004) Signalling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther 102: 37–46
Grandis JR, Tweardy DJ (1993) Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res 53: 3579–3584
Grisanti S, Amoroso V, Buglione M, Rosati A, Gatta R, Pizzocaro C, Ferrari VD, Marini G (2008) Cetuximab in the treatment of metastatic mucoepidermoid carcinoma of the salivary glands: a case report and review of literature. J Med Case Reports 2: 320–324
Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, Weeks L, Costello R, Posner M (2003) Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol 39: 724–727
Han SW, Kim HP, Jeon YK, Oh DY, Lee SH, Kim DW, Im SA, Chung DH, Heo DS, Bang YJ, Kim TY (2008) Mucoepidermoid carcinoma of lung: potential target of EGFR-directed treatment. Lung Cancer 61: 30–34
Handra-Luca A, Bilal H, Bertrand JC, Fouret P (2003) Extra-cellular signal-regulated ERK-1/ERK-2 pathway activation in human salivary gland mucoepidermoid carcinoma. Am J Pathol 163: 957–967
Handra-Luca A, Lamas G, Bertrand JC, Fouret P (2005) MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications. Am J Surg Pathol 29: 881–889
Hirsch FR, Varella-Garcia M, Bunn Jr PA, Di Maria MV, Veve R, Bremnes RM, Baron AE, Zeng C, Franklin WA (2003) Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol 21: 3798–3807
Hirsch FR, Varella-Garcia M, McCoy J, West H, Xavier AC, Gumerlock P, Bunn Jr PA, Franklin W, Crowley J, Gandara DR (2005) Increased epidermal growth factor receptor gene copy number detected by fluorescence in situ hybridization associates with increased sensitivity to gefitinib in patients with bronchioloalveolar carcinoma subtypes: a Southwest Oncology Group Study. J Clin Oncol 23: 6838–6845
Jilaveanu L, Zito C, Lee SJ, Nathanson KL, Camp RL, Rimm DL, Flaherty KT, Kluger HM (2009) Expression of sorafenib targets in melanoma patients treated with carboplatin, paclitaxel and sorafenib. Clin Cancer Res 15: 1076–1085
Kalyankrishna S, Grandis JR (2006) Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 24: 2666–2672
Kang SM, Kang HJ, Shin JH, Kim H, Shin DH, Kim SK, Kim JH, Chung KY, Kim SK, Chang J (2007) Identical epidermal growth factor receptor mutations in adenocarcinomatous and squamous cell carcinomatous components of adenosquamous carcinoma of the lung. Cancer 109: 581–587
Klapper LN, Kirschbaum MH, Sela M, Yarden Y (2000) Biochemical and clinical implications of ErbB/HER signaling network of growth factor receptors. Adv Cancer Res 77: 25–79
Lewis TS, Shapiro PS, Ahn NG (1998) Signal transduction through MAP kinase cascades. Adv Cancer Res 74: 49–139
Macarenco RS, Uphoff TS, Gilmer HF, Jenkins RB, Thibodeau SN, Lewis JE, Molina JR, Yang P, Aubry MC (2008) Salivary gland-type lung carcinomas: an EGFR immunohistochemical, molecular genetic, and mutational analysis study. Mod Pathol 21: 1168–1175
Nance MA, Seethala RR, Wang Y, Chiosea SI, Myers EN, Johnson JT, Lai SY (2008) Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 113: 2082–2089
Nicholson RI, Gee JMW, Harper ME (2001) EGFR and cancer prognosis. Eur J Cancer 37 (Suppl 4): S9–S15
Pochampalli MR, Bitler BG, Schroeder JA (2007) Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res 67: 6591–6598
Press MF, Pike MC, Hung G, Zhou JY, Ma Y, George J, Dietz-Band J, James W, Slamon DJ, Batsakis JG, El-Naggar AK (1994) Amplification and overexpression of HER-2/neu in carcinomas of the salivary gland: correlation with poor prognosis. Cancer Res 54: 5675–5682
Rossi G, Sartori G, Cavazza A, Tamberi S (2009) Mucoepidermoid carcinoma of the lung, response to EGFR inhibitors, EGFR and K-RAS mutations, and differential diagnosis. Lung Cancer 63: 159–160
Sartore-Bianchi A, Moroni M, Veronese S, Carnaghi C, Bajetta E, Luppi G, Sobrero A, Barone C, Cascinu S, Colucci G, Cortesi E, Nichelatti M, Gambacorta M, Siena S (2007) Epidermal growth factor receptor gene copy number and clinical outcome of metastatic colorectal cancer treated with panitumumab. J Clin Oncol 25: 3238–3245
Schroeder JA, Thompson MC, Gardner MM, Gendler SJ (2001) Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem 276: 13057–13064
Shang J, Shui Y, Sheng L, Wang K, Hu Q, Wei Q (2007) Epidermal growth factor receptor and human epidermal growth factor 2 expression in parotid mucoepidermoid carcinoma: possible implication for targeted therapy. Oncol Rep 19: 435–440
Sobin LH, Wittekind C (2002) TNM Classification of Malignant Tumours. American Joint Committee on Cancer and International Union Against Cancer (UICC), 6th edn, Willey and Sons: New York
Sone T, Kasahara K, Kimura H, Nishio K, Mizuguchi M, Nakatsumi Y, Shibata K, Waseda Y, Fujimura M, Nakao S (2007) Comparative analysis of EGFR mutations and gene amplification as predictors of gefitinig efficacy in Japanese patients with nonsmall cell lung cancer. Cancer 109: 1836–1844
Spiro RH (1986) Salivary neoplasms: overview of a 35-year experience with 2807 patients. Head Neck Surg 8: 177–184
Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, Lorimer I, Zhang T, Liu N, Daneshmand M, Marrano P, da Cunha Santos G, Lagarde A, Richardson F, Seymour L, Whitehead M, Ding K, Pater J, Shepherd FA (2005) Erlotinib in lung cancer – molecular and clinical predictors of outcome. N Engl J Med 353: 133–144
Yoo J, Robinson RA (2000a) H-ras gene mutations in salivary gland mucoepidermoid carcinomas. Cancer 88: 518–523
Yoo J, Robinson RA (2000b) ras Gene mutations in salivary gland tumours. Arch Pathol Lab Med 124: 836–839
Acknowledgements
We thank Elena Gonzalvo for performing the immunohistochemical techniques, and Gemma Laguna for secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lujan, B., Hakim, S., Moyano, S. et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer 103, 510–516 (2010). https://doi.org/10.1038/sj.bjc.6605788
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605788
Keywords
This article is cited by
-
Distinct histone H3 modification profiles correlate with aggressive characteristics of salivary gland neoplasms
Scientific Reports (2022)
-
Predictive value of FHIT, p27, and pERK1/ERK2 in salivary gland carcinomas: a retrospective study
Clinical Oral Investigations (2019)
-
Silymarin and its active component silibinin act as novel therapeutic alternatives for salivary gland cancer by targeting the ERK1/2-Bim signaling cascade
Cellular Oncology (2017)
-
Phosphorylated epidermal growth factor receptor expression and KRAS mutation status in salivary gland carcinomas
Clinical Oral Investigations (2016)
-
Gene expression profiling analysis of CRTC1-MAML2 fusion oncogene-induced transcriptional program in human mucoepidermoid carcinoma cells
BMC Cancer (2015)