Abstract
Background:
The role of adjuvant chemotherapy after resection of colorectal cancers (CRCs) is well understood for patients with stage-I or stage-III disease. Its efficacy for those with stage-II disease remains much less clear. Many investigators have sought to identify prognostic markers that might clarify which patients have the highest risk of recurrence and would, therefore, be most likely to benefit from chemotherapy. This systematic review examines evidence for the use of peripherally sampled, circulating tumour cells (CTCs) as such a prognostic marker.
Methods:
A comprehensive literature search was used to identify studies reporting on the significance of CTCs in the postoperative blood of CRC patients.
Results:
Fourteen studies satisfied the inclusion criteria. Six of the nine studies that took blood samples 24 h or more postoperatively found detection of postoperative CTCs to be an independent predictor of cancer recurrence.
Conclusion:
The presence of CTCs in peripheral blood at least 24 h after resection of CRCs is an independent prognostic marker of recurrence. Further studies are needed to clarify the optimal time point for blood sampling and determine the benefit of chemotherapy in CTC-positive patients with stage-II disease.
Similar content being viewed by others
Main
In modern clinical practice, the accepted treatment for non-metastatic colorectal cancer (CRC) is tumour resection, with or without adjuvant chemotherapy depending on tumour stage. For stage-I cancers, surgical resection is generally curative without the need for chemotherapy, whereas for stage-III (node-positive) cancers, adjuvant chemotherapy has been shown to significantly improve outcomes (1990). For patients with stage-II (node-negative) cancers, however, the benefits of adjuvant chemotherapy remain much less clear. For these patients, any potential benefits are often outweighed by the risks and side-effects of treatment (Benson et al, 2004). For this reason, the international QUASAR trial was set up to assess the efficacy of adjuvant chemotherapy in this patient group. QUASAR concluded that although adjuvant chemotherapy may improve survival of patients with stage-II CRC, the absolute improvement in 5-year survival was minimal at only 3.6% (Quasar Collaborative Group, 2007).
With recurrence rates (RRs) being highly variable for patients with stage-II disease, investigators have sought to differentiate between those at high risk and those at low risk of recurrence. By distinguishing these two subgroups it might be possible to identify those patients most likely to benefit from adjuvant chemotherapy and target treatment accordingly. Previously established prognostic markers for cancers at high risk of recurrence include local tumour extent, regional lymph node metastasis, blood or lymphatic vessel invasion, residual tumour after curative surgery, and preoperative elevation of carcinoembryonic antigen (CEA) (Compton et al, 2000). More recently, molecular detection of circulating tumour cells (CTCs) has also been investigated as a potential prognostic marker.
Although previous analyses have demonstrated some prognostic significance for CTC levels in portal blood sampled intraoperatively (Katsuno et al, 2008), studies assessing the significance of preoperative CTC levels have shown little predictive value in terms of clinical outcome (Wharton et al, 1999; Tsavellas et al, 2001). Other reviews have also assessed the significance of CTC levels, but these have included relatively few studies with information on postoperative CTC detection (Sergeant et al, 2008), and early findings have suggested that this postoperative detection of CTCs may actually offer greatest prognostic value. We therefore undertook a systematic review of the evidence for the use of peri- and postoperative CTC detection in predicting the outcome following surgical resection of colorectal cancers (CRCs).
The objectives of this systematic review were (1) to examine current literature and clarify the prognostic significance of peripherally sampled CTCs after resection of non-metastatic CRCs; (2) to identify those markers most likely to be of use in risk-stratification of CRC patients after potentially curative surgery; and (3) to identify the most appropriate directions for further research.
Materials and methods
The measurement tool for ‘assessment of multiple systematic reviews’ (AMSTAR) was used as a reference. This tool consists of 11 items and has good face and content validity for measuring the methodological quality of systematic reviews (Shea et al, 2007).
Literature search
A literature search was performed using multiple electronic search engines, including Medline (using PubMed), the Cochrane Database, Ovid, and Google Scholar. Studies reporting on the molecular detection of CTCs in postoperative peripheral blood and its effect on prognosis in CRC (last search date 1st December 2009) were identified. The following keywords were used for the search: ‘colon’, ‘rectal’, ‘colorectal cancer’, ‘circulating cells’, ‘prognosis’, ‘mRNA’, ‘PCR’, ‘-breast’, ‘-gastric’. The ‘related articles’ function in PubMed and Google Scholar was also used to identify additional articles. References of the articles identified were also searched for by title and then subsequent abstract review.
Eligibility criteria and data extraction
All published studies reporting on the effect of CTCs in postoperative peripheral blood in CRC on prognosis were considered. There were no restrictions made on the type of study.
Data were extracted on author, year of publication, study design, technical aspects of the studies, and outcomes. All data were extracted by two reviewers (C Kim and G Peach), and any discrepancies were resolved by consensus from all authors. Restrictions were made to papers published in the English language.
Inclusion and exclusion criteria
To be included in the analysis, studies had to (1) include patients undergoing curative resection of CRC; (2) include peri- or postoperative CTC detection; (3) report on at least one of the outcome measures listed in the next section; and (4) include peripheral blood samples. When two studies were reported by the same institution and/or authors, both were included in the analysis.
Studies were excluded from the analysis if (1) outcomes of interest were not reported; (2) it was impossible to extract or calculate the necessary data from the published results; (3) it only reported on the preoperative sampling of peripheral blood; and (4) only portal and/or mesenteric blood sampling was undertaken.
Outcomes of interest and definitions
The outcomes of interest were any type of prognostic data, including: disease-free survival (DFS), overall survival (OS), RR, cancer-specific survival, and odds and hazards ratios (HRs).
Data analysis
Raw data on outcomes of interest were collected and tabulated. The data were stored on Microsoft Office Excel.
Results
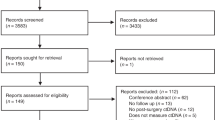
Six hundred and sixty-four articles were identified using the above keywords. Title and abstract review resulted in the exclusion of 618 articles that did not address the molecular detection of CTCs and prognosis in CRC. Forty-six references were assessed in full, and a further 32 studies were excluded (Figure 1). Eight studies were excluded as they reported only on preoperative blood sampling (Bessa et al, 2001; Fujita et al, 2001; Wong et al, 2001; Zhang et al, 2005; Douard et al, 2006; Iinuma et al, 2006; Wang et al, 2006; Friederichs et al, 2007). Nine studies were excluded as they contained data from which outcomes could not be extracted, of which four were excluded as they combined positive results of molecular detection of multiple samples and their effect on prognosis (Hardingham et al, 2000; Guller et al, 2002; Bosch et al, 2003; Koyanagi et al, 2008), and five were excluded as it was impossible to extract the data for outcomes of interest (White and Griffiths, 1976; Funaki et al, 1998; Wyld et al, 1998; Conzelmann et al, 2005; Wang et al, 2007). Five studies were excluded as they were reviews or systematic reviews, which did not report on prognostic outcomes (Tsavellas et al, 2001; Sleijfer et al, 2007; Riethdorf et al, 2008; Sergeant et al, 2008; Tsouma et al, 2008). Ten studies were excluded as they did not report on prognostic outcomes (Denis et al, 1997; Nakamori et al, 1997; Douard et al, 2001; Guadagni et al, 2001; Patel et al, 2002; Silva et al, 2002; Yokoyama and Yamaue, 2002; Schuster et al, 2004; Yeh et al, 2006; Lagoudianakis et al, 2009). The outcomes measured in the 14 studies included in this review were too heterogeneous with regards to the methodology of data collection to allow quantitative analysis. A systematic review was therefore undertaken.
Study characteristics
The 14 studies included here comprised 1841 patients in total, with the median number of patients in each study being 99.5 (range 42–438) (Taniguchi et al, 2000; Yamaguchi et al, 2000; Ito et al, 2002; Bessa et al, 2003; Chen et al, 2004; Sadahiro et al, 2005, 2007; Katsumata et al, 2006; Koch et al, 2006; Lloyd et al, 2006; Allen-Mersh et al, 2007; Uen et al, 2007, 2008; Wang et al, 2007).
Of the 14 studies included, eight undertook blood sampling peri-operatively (Taniguchi et al, 2000; Yamaguchi et al, 2000; Ito et al, 2002; Chen et al, 2004; Sadahiro et al, 2005; Katsumata et al, 2006; Koch et al, 2006; Lloyd et al, 2006), four undertook sampling approximately 24 h after resection (Bessa et al, 2003; Koch et al, 2006; Lloyd et al, 2006; Allen-Mersh et al, 2007), and six undertook sampling between 24 h and 14 days after resection (Chen et al, 2004; Allen-Mersh et al, 2007; Sadahiro et al, 2007; Uen et al, 2007, 2008; Wang et al, 2007).
Tumour stage
The studies examined in this review included patients with various different stages of disease. Seven investigated the role of CTC in patients with stage-I to stage-III disease undergoing potentially curative surgery (Ito et al, 2002; Bessa et al, 2003; Sadahiro et al, 2005, 2007; Allen-Mersh et al, 2007; Wang et al, 2007; Uen et al, 2008); three studied CTC in patients with early CRC (of which two investigated stage-II disease alone (Koch et al, 2006; Uen et al, 2007) and one investigated stage-I and stage-II disease (Lloyd et al, 2006)); and four papers included patients with stage-I to stage-IV disease (Taniguchi et al, 2000; Yamaguchi et al, 2000; Chen et al, 2004; Katsumata et al, 2006).
Blood sampling site
Of the 14 studies included in our analysis, 10 involved sampling of peripheral blood only (Ito et al, 2002; Bessa et al, 2003; Chen et al, 2004; Katsumata et al, 2006; Lloyd et al, 2006; Allen-Mersh et al, 2007; Sadahiro et al, 2007; Uen et al, 2007, 2008; Wang et al, 2007); 1 used peripheral and portal blood samples (Sadahiro et al, 2005); 1 used peripheral and mesenteric samples (Yamaguchi et al, 2000); 1 used peripheral, portal and mesenteric samples (Taniguchi et al, 2000); and 1 used samples of central venous blood (Koch et al, 2006).
CTC detection methods
Thirteen of the 14 included studies used identification of specific mRNA to detect the presence of CTCs. Eleven of these used reverse transcriptase-PCR (RT-PCR) to detect tumour cells in the sampled blood (Taniguchi et al, 2000; Yamaguchi et al, 2000; Ito et al, 2002; Bessa et al, 2003; Chen et al, 2004; Sadahiro et al, 2005, 2007; Katsumata et al, 2006; Koch et al, 2006; Allen-Mersh et al, 2007; Uen et al, 2007) whereas the other two, both from the same unit, used a membrane array (Wang et al, 2007; Uen et al, 2008). The remaining study used immunocytochemical staining of circulating cells after immunomagnetic purification (Lloyd et al, 2006).
Seven of the included studies used a single marker to detect CTCs, with the chosen marker being CEA mRNA (n=5), cytokeratin-20 (CK20), or guanylyl cyclase (GCC) (Taniguchi et al, 2000; Ito et al, 2002; Bessa et al, 2003; Chen et al, 2004; Sadahiro et al, 2005, 2007; Koch et al, 2006). The other studies used multiple markers, with an overall CTC-positive result usually being determined by detection of more than one marker.
CTC detection rates
There was a mean detection rate of 33.4% (±3.6 s.e.m.). There were no differences between studies that sampled peri-operatively, early postoperatively, or late postoperatively, nor were there any differences between studies that included only early-stage disease, curative patients only, and patients at all stages. Furthermore, there was no demonstrable difference in detection rate between studies that used one, two, or multiple markers. Direct comparison between the different cellular markers could not be made due to differences in methodology.
In the three series that only investigated patients with early-stage disease (i.e., stage-I and/or stage-II) the detection rates were variable. Koch et al (2006) and Uen et al (2007) reported positivity rates of 34 and 27%, respectively, whereas the paper by Lloyd et al (2006) only reported a positive detection rate of less than 5% in the peripheral blood postoperatively. This paper described the use of an immunobead technique to purify blood cells, which may account for any differences.
Patient follow-up
Median follow-up (where stated) ranged from 36 to 68 months. In the 10 papers that clearly stated the length of follow-up, there was no identifiable relationship between length of follow-up and the reported level of CTC significance.
Prognostic influence of CTCs
Peri-operative sampling
In the first of the studies to take peri-operative samples (Table 1), Taniguchi et al (2000) took samples of both peripheral and portal blood intra-operatively (before mobilisation of the tumour) and examined them for CEA mRNA using RT-PCR. Thirty-four percent of patients showed CTC positivity in peripheral blood and after potentially curative surgery; this group of patients had a lower DFS (50 vs 74% at 2 years; P<0.01) than patients without detectable CTC. Conversely, although Sadahiro et al (2005) also took peripheral blood samples intra-operatively (pre-resection) and also used a CEA mRNA protocol, they failed to show any link between CTC positivity and tumour recurrence or survival. Ito et al also tested for CEA mRNA using RT-PCR, but took samples immediately after operation. They showed positivity rates of 37% and showed that those patients who were CTC-positive once again had reduced DFS (77 vs 92% at 58 months; P=0.0296).
All other studies that took peri-operative samples used detection of multiple cellular markers. Yamaguchi et al (2000) showed that positivity for both CEA and CK20 in blood samples obtained intra-operatively was associated with a trend towards decreased survival (60 vs 85% at 1 year), although this was not a statistically significant difference (P=0.06). Katsumata et al (2006) chose to use CEA, CK19, and CK20 mRNA as markers to detect CTCs in patients with stage-I to stage-IV disease undergoing surgery. They found both CK19 and CEA positivity to be unrelated to tumour progression. However, they also showed that whereas CK20 expression had no correlation with local tumour recurrence, it did correlate with the presence of lymph node metastasis (P=0.037), and that 5-year survival in the CK20-positive group was 65.2 vs 87.5% in the CK-negative group (P=0.048). Nonetheless, CTC positivity as determined by CK20 was not found to be an independent prognostic marker. This apparent discrepancy is probably related to CTC positivity reflecting stage (as shown by its correlation with lymph node metastases) rather than prognosis.
Lloyd et al (2006) used CK20, CEA ephrin-B4, laminin-γ2, and matrilysin mRNA as markers of CTC in peripheral samples taken preoperatively and intra-operatively (as well as post-operatively – see below). They did not show any significant correlation between CTC positivity and survival although mean survival was only 36.5 months in CTC-positive patients as compared with 76 months in negative patients. It should be noted that although patients in this study had early disease (stage-I and stage-II only), the number to test positive for any marker postoperatively was very low (4%) compared with other papers in this review. Furthermore, none of the patients were positive for circulating CEA mRNA preoperatively, in contrast to other studies discussed here.
Finally, Chen et al (2004) used GCC as a marker of CTCs in peripheral blood samples taken pre-, intra-, and postoperatively. Whereas CTC positivity in preoperative and intra-operative samples did not correlate with prognosis, patients who were CTC positive in samples taken 14 days postoperatively had significantly lower DFS and OS (see below).
Early postoperative sampling (0–48 h)
In the first study that looked at postoperative sampling (Table 2), Bessa et al (2003) investigated 66 patients with stage-I to stage-III disease undergoing potentially curative surgery. They used RT-PCR to detect CEA mRNA in peripheral blood both before and 24 h after surgery. No data were presented in relation to the prognostic significance of preoperative CTC positivity. Postoperative samples showed a 55% positivity rate but RR were almost identical in both CEA mRNA-positive and negative groups (22 vs 23%, respectively, at 36 months). Similarly, postoperative CTC positivity did not influence OS. After adjusting for TNM stage, the probability of RRs and OS rates were also the same in both groups. In addition to taking preoperative and intra-operative samples (see above), Lloyd et al (2006) also took peripheral blood samples the morning after surgery. Unlike Bessa et al, they found that mean survival was reduced in patients who were CTC-positive at this time point, but this was not a statistically significant result (mean survival 42.6 months vs 75.9; P=0.6). However, it is interesting to note that in this study the postoperative positivity rate was very low (4.2%), just as it had been in their preoperative samples – even though high positivity rates might be expected as positivity was defined as presence of any one of the five markers used.
Allen-Mersh et al (2007) took peripheral samples and used RT-PCR to detect both CK20 and CEA. Samples were taken preoperatively, 24 h after resection, and at 1 week after operation (see below). They found no significant difference in outcome between patients who were CTC-positive preoperatively (as defined by presence of either marker) and those who were CTC-negative preoperatively. However, patients who were CTC-positive 24 h postoperatively had much poorer DFS (56 vs 90% at 2 years; P<0.001). These results are supported by the findings of Koch et al (2006) who took samples of central venous blood at 24 h after resection (as well as peri-operatively – see above). This group investigated only patients who had stage-II disease and CK20 was used as the detection marker. They showed that CTC positivity at 24 h was associated with reduced 5-year relapse-free survival (70 vs 93%; P=0.003) and disease-specific survival (77 vs 100%; P=0.0006), the latter regardless of whether patients had received chemotherapy or not. Furthermore, multivariate analysis confirmed that identification of CTC tumour cell in the peripheral blood at 24 h after surgery was an independent predictor of relapse and disease-specific mortality (HR 2.4 (1.3–5.3); P=0.008, and HR 6.4 (2.2–34); P=0.0003, respectively).
Late postoperative sampling (>48 h)
A number of studies have also looked at the role of CTCs persisting more than 48 h after surgery (Table 3). Sadahiro et al (2007) used RT-PCR to detect CEA in peripheral blood samples taken 7–10 days after resection from patients with stage-I to stage-III disease. The overall rate of postoperative CTC positivity was 22% and CTC-positive patients showed significantly poorer DFS (51 vs 70% at 5 years; P=0.007) and OS (65 vs 80% at 5 years; P=0.04) than CTC-negative patients. They also found that even among patients with stage-I disease, RRs were much higher in the CEA mRNA-positive group (45 vs 22%; P=0.003). Overall, they were able to show that CEA mRNA positivity was a significant independent risk factor for tumour recurrence (RR 2.29 (1.30–4.02)) but not for OS (RR 1.81 (0.94–3.50)).
Three studies by the same group (Uen et al, 2007, 2008; Wang et al, 2007) used a membrane array technique to detect multiple cellular markers. These were CK20, CK19, CEA, and human telomerase reverse transcriptase (hTERT), and CTC positivity was defined as presence of all four of these markers. In 194 patients with stage-II disease undergoing curative surgery, 27.5% were positive for CTC at least one week after surgery. Almost 85% of positive patients relapsed whereas only 7.8% of patients negative for CTC developed recurrence (Uen et al, 2007). Positivity was shown to be a predictor of relapse (HR 38.6, 13.9–106.9), greater than T-stage or vascular invasion alone. Similarly, when looking at all patients undergoing curative surgery (i.e., those with stage-I to stage-III disease), the same authors again showed that CTC positivity at 1 week after resection was a prognostic indicator for recurrence (P<0.001; HR 29.5 (10.3–87.8)). Worse DFS was also seen in patients with CTC detected postoperatively (P<0.001). This group also looked at the prognostic effect of CTC detected 4 weeks after surgery using the same membrane array for the described markers and again showed significant association between positive CTC and recurrence (HR 18.7 (5.6–112.8)).
Although Chen et al (2004) were unable to show any prognostic significance of preoperative and intra-operative CTC levels (see above), they too found late postoperative CTC levels to be a highly significant indicator of outcome. At 14 days after resection, patients who were CTC-positive (defined as more than 102 CTC per 106 nucleated blood cells) had much worse DFS (50 vs 94% at 36 months; P=0.001) and OS (65 vs 94% at 36 months; P=0.039) than those who were CTC-negative. No multivariate analysis was performed to investigate whether high CTC load was an independent risk factor.
Allen-Mersh et al (2007) not only took samples preoperatively and at 24 h after operation (see above), but also at 1 week after resection. At this time point they were unable to show any difference in prognosis between the CTC-positive and CTC-negative groups, although this is most probably due to the fact that there were very few positive results by 1 week after the operation and this would render any differences between the two groups statistically insignificant.
Discussion
Staging of disease with Dukes' or TNM systems is generally used to help predict recurrence and cancer-specific survival, and identify patients who may benefit from adjuvant chemotherapy. As indiscriminate use of chemotherapy for patients with stage-II disease results in minimal reduction in mortality, identification of a new prognostic marker may help to determine which patients would benefit most. Although the majority of the studies included in this review did not limit their investigations to patients with stage-II disease, their findings may provide good basis for larger subsequent trials in this patient group.
The papers presented here did not show peri-operative CTC levels to be of value in predicting recurrence of CRC. This echoes the findings of most previous studies looking at preoperative markers, and whereas Koyanagi et al (2008) found preoperative detection of CTCs to be an independent prognostic indicator, this has not been supported by earlier reviews (Wharton et al, 1999; Tsavellas et al, 2001). Some also noted differences in DFS between those who were CTC-positive preoperatively and those who were CTC-negative. This may simply be a reflection of tumour stage however, as several of these papers found CTC detection rate to be dependent on other clinicopathological characteristics (such as T-stage and lymph node status) that are known to have prognostic significance.
Six of the nine papers investigating the prognostic role of CTCs persisting 24 h or more after resection found CTC detection to be an independent predictor of cancer recurrence (Koch et al, 2006; Allen-Mersh et al, 2007; Sadahiro et al, 2007; Uen et al, 2007, 2008; Wang et al, 2007). Furthermore, 2 out of 4 papers using early postoperative sampling showed that CTC detected as early as 24 h postoperatively had significant influence on recurrence (Koch et al, 2006; Allen-Mersh et al, 2007). Koch et al (2006) also showed that detection of CTC predicted poorer cancer specific survival. Unlike Koch et al, Lloyd et al (2006) did not show any relationship between CTC detection and cancer-specific outcomes. However, it is interesting to note that although patients in this study had early disease (stage-I and stage-II only), positivity rate for any of the five markers used was very low at 4.2% and only 1 out of 113 patients was positive for circulating CEA mRNA postoperatively. This is in stark contrast to the other studies, which showed positivity rates between 22 and 62.9%. Even the studies by Koch et al (2006) and Uen et al (2007) (which included stage-II patients only) had positivity rates of 34 and 27.5%, respectively. This may be explained by the unique immunomagnetic bead methodology that Lloyd et al used to isolate cells from the blood. With such low rates of CTC detection, it is highly unlikely that they would have been able to show a significant association between persisting CTC and recurrence.
Bessa et al (2003) also failed to show any association between CTC positivity at 24 h and recurrence. However, it is once again interesting to note that in this study all 28 patients with stage-II disease received 5-fluorouracil-based adjuvant chemotherapy, unlike in the studies by Koch et al (2006) and Allen-Mersh et al (2007), where very few node-negative patients received chemotherapy (6 and 13% of patients, respectively). This use of chemotherapy for patients studied by Bessa et al may well have improved the outcomes of CTC-positive patients with stage-II disease, thereby masking any potential difference between the two groups. Furthermore, false-positive and false-negative rates were not presented in this study and may have influenced their results.
The presence of free CTC is dependent on two processes: dissemination of cancer cells into the circulation and subsequent elimination of those cells. Dissemination is thought to occur from both the primary tumour site and from occult micrometastases, such as bone marrow, lung, or liver (Pantel and Brakenhoff, 2004). Surgical manipulation may increase CTC release from the primary tumour during surgery and make peri- or early postoperative sampling unreliable. This could explain why peri-operative sampling has not been shown to be of prognostic significance (as found in this review) and why few previous studies have been able to show a role for preoperative CTC. In terms of dissemination from micrometastases, ongoing release of CTC from these sites may explain why some patients have persistently high CTC levels even after resection. This might contribute to the apparent association between high postoperative CTC levels and poor prognosis, as high CTC levels could signify more systemically advanced disease.
Elimination of CTC may also occur by a number of pathways, including anoikis, mechanical stress, microembolie (Geiger and Peeper, 2009), and possibly immunological eradication (Johansson et al, 2008). Although it has previously been suggested that most CTC are cleared from the circulation within 24 h of tumour resection (Fidler, 1970; Patel et al, 2002), persisting CTCs may have increased resistance to these degradation processes and therefore show greater metastatic potential (Song et al, 2001). This would also be in keeping with the demonstrated correlation between long-surviving tumour cells and poor prognosis. The significance of rate of CTC clearance was not generally assessed in detail in the papers analysed here. Nonetheless, the demonstrated correlation between persisting CTC positivity and poor prognosis may suggest that more rapid clearance would be associated with better prognosis. This is supported in a previous study by Patel et al (2002), which found that clearance of circulating tumour cells within 24 h of CRC excision was greatest in patients with stage-I and stage-II disease.
For CTC testing to be used clinically as a prognostic indicator, it is also important to establish the most reliable time point for blood sampling. The evidence presented here suggests that when testing is undertaken at least 24 h after surgery, presence of CTC has a prognostic role: Allen-Mersh et al (2007) and Koch et al (2006) both showed significance at 24 h, with an additional five papers showing a clear role for testing between 7 and 14 days postoperatively. However, as direct comparison between these time points has not yet been undertaken, the optimal timing for collection of blood samples has yet to be clarified.
Whereas use of multiple cellular markers might be expected to increase sensitivity and specificity, detection rates were found to be similar in each series and across all markers. The studies from Taiwan (Chen et al, 2004; Uen et al, 2007, 2008; Wang et al, 2007) used an RNA array to detect four different markers, whereas others showed a prognostic role for CTC using either CEA or CK20 mRNA (or both). A number of additional factors may also affect the results. These include such things as use of chemotherapy and differences in RT-PCR methodology, which may vary due to the method of blood preparation or the time lapse between sampling and processing. These are not examined in more detail here, as meta-analysis was not possible due to heterogeneity of existing data. One might also expect variations in study size to affect the degree to which CTC significance could be shown. However, whereas the more highly powered studies did indeed tend to show greatest significance, studies with relatively few patients (e.g., Koch et al, 2006) were also able to show prognostic significance of CTC.
Conclusion
The presence of CTCs in peripheral blood at least 24 h after resection of CRCs is an independent prognostic marker of recurrence. However, further studies are needed to clarify the optimal time point for postoperative blood sampling, and to identify the most reliable cellular marker for measurement of CTC level. The evidence presented here provides a possible basis for future large-scale, multi-centre trials with more unified methodology. By including a greater number of patients with stage-II disease, it may be possible to clarify the significance of CTC within this subgroup and more accurately identify those patients most likely to benefit from chemotherapy.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Allen-Mersh TG, McCullough TK, Patel H, Wharton RQ, Glover C, Jonas SK (2007) Role of circulating tumour cells in predicting recurrence after excision of primary colorectal carcinoma. Br J Surg 94: 96–105
Benson III AB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, Brouwers M, Charette M, Haller DG (2004) American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 22: 3408–3419
Bessa X, Elizalde JI, Boix L, Pinol V, Lacy AM, Salo J, Pique JM, Castells A (2001) Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology 120: 1084–1092
Bessa X, Pinol V, Castellvi-Bel S, Piazuelo E, Lacy AM, Elizalde JI, Pique JM, Castells A (2003) Prognostic value of postoperative detection of blood circulating tumor cells in patients with colorectal cancer operated on for cure. Ann Surg 237: 368–375
Bosch B, Guller U, Schnider A, Maurer R, Harder F, Metzger U, Marti WR (2003) Perioperative detection of disseminated tumour cells is an independent prognostic factor in patients with colorectal cancer. Br J Surg 90: 882–888
Chen WS, Chung MY, Liu JH, Liu JM, Lin JK (2004) Impact of circulating free tumor cells in the peripheral blood of colorectal cancer patients during laparoscopic surgery. World J Surg 28: 552–557
Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, Nielsen ML, Sargent DJ, Taylor CR, Welton M, Willett C (2000) Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124: 979–994
Conzelmann M, Linnemann U, Berger MR (2005) Molecular detection of clinical colorectal cancer metastasis: how should multiple markers be put to use? Int J Colorectal Dis 20: 137–146
Denis MG, Lipart C, Leborgne J, LeHur PA, Galmiche JP, Denis M, Ruud E, Truchaud A, Lustenberger P (1997) Detection of disseminated tumor cells in peripheral blood of colorectal cancer patients. Int J Cancer 74: 540–544
Douard R, Le Maire V, Wind P, Sales JP, Dumas F, Fayemendi L, Landi B, Benichou J, Cugnenc PH, Gayral F, Loric S (2001) Carcinoembryonic gene member 2 mRNA expression as a marker to detect circulating enterocytes in the blood of colorectal cancer patients. Surgery 129: 587–594
Douard R, Wind P, Sales JP, Landi B, Berger A, Benichou J, Gayral F, Loric S, Cugnenc PH (2006) Long-term prognostic value of detection of circulating colorectal cancer cells using CGM2 reverse transcriptase-polymerase chain reaction assay. Surgery 139: 556–562
Fidler IJ (1970) Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst 45: 773–782
Friederichs J, Gertler R, Rosenberg R, Dahm M, Nekarda H, Holzmann B, Siewert JR (2007) Correlation of CK-20-positive cells in peripheral venous blood with serum CEA levels in patients with colorectal carcinoma. World J Surg 31: 2329–2334
Fujita S, Kudo N, Akasu T, Moriya Y (2001) Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis 16: 141–146
Funaki NO, Tanaka J, Ohshio G, Onodera H, Maetani S, Imamura M (1998) Cytokeratin 20 mRNA in peripheral venous blood of colorectal carcinoma patients. Br J Cancer 77: 1327–1332
Geiger TR, Peeper DS (2009) Metastasis mechanisms. Biochim Biophys Acta 1796: 293–308
Guadagni F, Kantor J, Aloe S, Carone MD, Spila A, D'Alessandro R, Abbolito MR, Cosimelli M, Graziano F, Carboni F, Carlini S, Perri P, Sciarretta F, Greiner JW, Kashmiri SV, Steinberg SM, Roselli M, Schlom J (2001) Detection of blood-borne cells in colorectal cancer patients by nested reverse transcription-polymerase chain reaction for carcinoembryonic antigen messenger RNA: longitudinal analyses and demonstration of its potential importance as an adjunct to multiple serum markers. Cancer Res 61: 2523–2532
Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, Harder F, Heberer M, Marti WR (2002) Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg 236: 768–775; discussion 775–766
Hardingham JE, Hewett PJ, Sage RE, Finch JL, Nuttall JD, Kotasek D, Dobrovic A (2000) Molecular detection of blood-borne epithelial cells in colorectal cancer patients and in patients with benign bowel disease. Int J Cancer 89: 8–13
Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, Adachi M, Mori M, Sasako M (2006) Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol 28: 297–306
Ito S, Nakanishi H, Hirai T, Kato T, Kodera Y, Feng Z, Kasai Y, Ito K, Akiyama S, Nakao A, Tatematsu M (2002) Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer Lett 183: 195–203
Johansson M, Denardo DG, Coussens LM (2008) Polarized immune responses differentially regulate cancer development. Immunol Rev 222: 145–154
Katsumata K, Sumi T, Mori Y, Hisada M, Tsuchida A, Aoki T (2006) Detection and evaluation of epithelial cells in the blood of colon cancer patients using RT-PCR. Int J Clin Oncol 11: 385–389
Katsuno H, Zacharakis E, Aziz O, Rao C, Deeba S, Paraskeva P, Ziprin P, Athanasiou T, Darzi A (2008) Does the presence of circulating tumor cells in the venous drainage of curative colorectal cancer resections determine prognosis? A meta-analysis. Ann Surg Oncol 15: 3083–3091
Koch M, Kienle P, Kastrati D, Antolovic D, Schmidt J, Herfarth C, von Knebel Doeberitz M, Weitz J (2006) Prognostic impact of hematogenous tumor cell dissemination in patients with stage II colorectal cancer. Int J Cancer 118: 3072–3077
Koyanagi K, Bilchik AJ, Saha S, Turner RR, Wiese D, McCarter M, Shen P, Deacon L, Elashoff D, Hoon DS (2008) Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res 14: 7391–7396
Lagoudianakis EE, Kataki A, Manouras A, Memos N, Papadima A, Derventzi A, Zografos G, Papadopoulos S, Katergiannakis V, Konstadoulakis MM (2009) Detection of epithelial cells by RT-PCR targeting CEA, CK20, and TEM-8 in colorectal carcinoma patients using OncoQuick density gradient centrifugation system. J Surg Res 155: 183–190
Lloyd JM, McIver CM, Stephenson SA, Hewett PJ, Rieger N, Hardingham JE (2006) Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res 12: 417–423
Nakamori S, Kameyama M, Furukawa H, Takeda O, Sugai S, Imaoka S, Nakamura Y (1997) Genetic detection of colorectal cancer cells in circulation and lymph nodes. Dis Colon Rectum 40: S29–S36
NIH consensus conference (1990) Adjuvant therapy for patients with colon and rectal cancer. JAMA 264: 1444–1450
Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4: 448–456
Patel H, Le Marer N, Wharton RQ, Khan ZA, Araia R, Glover C, Henry MM, Allen-Mersh TG (2002) Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg 235: 226–231
Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ (2007) Adjuvant chemotherapy vs observation in patients with colorectal cancer: a randomised study. Lancet 370: 2020–2029
Riethdorf S, Wikman H, Pantel K (2008) Review: biological relevance of disseminated tumor cells in cancer patients. Int J Cancer 123: 1991–2006
Sadahiro S, Suzuki T, Ishikawa K, Saguchi T, Maeda Y, Yasuda S, Makuuchi H, Yurimoto S, Murayama C (2005) Detection of carcinoembryonic antigen messenger RNA-expressing cells in portal and peripheral blood during surgery does not influence relapse in colorectal cancer. Ann Surg Oncol 12: 988–994
Sadahiro S, Suzuki T, Maeda Y, Yurimoto S, Yasuda S, Makuuchi H, Kamijo A, Murayama C (2007) Detection of carcinoembryonic antigen messenger RNA-expressing cells in peripheral blood 7 days after curative surgery is a novel prognostic factor in colorectal cancer. Ann Surg Oncol 14: 1092–1098
Schuster R, Max N, Mann B, Heufelder K, Thilo F, Grone J, Rokos F, Buhr HJ, Thiel E, Keilholz U (2004) Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer 108: 219–227
Sergeant G, Penninckx F, Topal B (2008) Quantitative RT-PCR detection of colorectal tumor cells in peripheral blood – a systematic review. J Surg Res 150: 144–152
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM (2007) Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7: 10
Silva JM, Rodriguez R, Garcia JM, Munoz C, Silva J, Dominguez G, Provencio M, Espana P, Bonilla F (2002) Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut 50: 530–534
Sleijfer S, Gratama JW, Sieuwerts AM, Kraan J, Martens JW, Foekens JA (2007) Circulating tumour cell detection on its way to routine diagnostic implementation? Eur J Cancer 43: 2645–2650
Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U (2001) Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer 85: 1047–1054
Taniguchi T, Makino M, Suzuki K, Kaibara N (2000) Prognostic significance of reverse transcriptase-polymerase chain reaction measurement of carcinoembryonic antigen mRNA levels in tumor drainage blood and peripheral blood of patients with colorectal carcinoma. Cancer 89: 970–976
Tsavellas G, Patel H, Allen-Mersh TG (2001) Detection and clinical significance of occult tumour cells in colorectal cancer. Br J Surg 88: 1307–1320
Tsouma A, Aggeli C, Pissimissis N, Lembessis P, Zografos GN, Koutsilieris M (2008) Circulating tumor cells in colorectal cancer: detection methods and clinical significance. Anticancer Res 28: 3945–3960
Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, Chi CW, Wang JY (2007) Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg 246: 1040–1046
Uen YH, Lu CY, Tsai HL, Yu FJ, Huang MY, Cheng TL, Lin SR, Wang JY (2008) Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I–III colorectal cancer after curative resection. Ann Surg Oncol 15: 2120–2128
Wang JY, Lin SR, Wu DC, Lu CY, Yu FJ, Hsieh JS, Cheng TL, Koay LB, Uen YH (2007) Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res 13: 2406–2413
Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, Lin SR (2006) Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis. World J Surg 30: 1007–1013
Wharton RQ, Jonas SK, Glover C, Khan ZA, Klokouzas A, Quinn H, Henry M, Allen-Mersh TG (1999) Increased detection of circulating tumor cells in the blood of colorectal carcinoma patients using two reverse transcription-PCR assays and multiple blood samples. Clin Cancer Res 5: 4158–4163
White H, Griffiths JD (1976) Circulating malignant cells and fibrinolysis during resection of colorectal cancer. Proc R Soc Med 69: 467–469
Wong IH, Yeo W, Chan AT, Johnson PJ (2001) Quantitative relationship of the circulating tumor burden assessed by reverse transcription-polymerase chain reaction for cytokeratin 19 mRNA in peripheral blood of colorectal cancer patients with Dukes' stage, serum carcinoembryonic antigen level and tumor progression. Cancer Lett 162: 65–73
Wyld DK, Selby P, Perren TJ, Jonas SK, Allen-Mersh TG, Wheeldon J, Burchill SA (1998) Detection of colorectal cancer cells in peripheral blood by reverse-transcriptase polymerase chain reaction for cytokeratin 20. Int J Cancer 79: 288–293
Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S (2000) Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg 232: 58–65
Yeh CS, Wang JY, Wu CH, Chong IW, Chung FY, Wang YH, Yu YP, Lin SR (2006) Molecular detection of circulating cancer cells in the peripheral blood of patients with colorectal cancer by using membrane array with a multiple mRNA marker panel. Int J Oncol 28: 411–420
Yokoyama S, Yamaue H (2002) Prediction of distant metastasis by using reverse transcriptase-polymerase chain reaction for epithelial and variant CD44 mRNA in the peripheral blood of patients with colorectal cancer. Arch Surg 137: 1069–1073
Zhang XW, Yang HY, Fan P, Yang L, Chen GY (2005) Detection of micrometastasis in peripheral blood by multi-sampling in patients with colorectal cancer. World J Gastroenterol 11: 436–438
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Peach, G., Kim, C., Zacharakis, E. et al. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer 102, 1327–1334 (2010). https://doi.org/10.1038/sj.bjc.6605651
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605651
Keywords
This article is cited by
-
Regional anesthesia might reduce recurrence and metastasis rates in adult patients with cancers after surgery: a meta-analysis
BMC Anesthesiology (2024)
-
Postoperative serum interleukin-6 levels correlate with survival in stage I-III colorectal cancer
BMC Gastroenterology (2023)
-
Prognostic factors in patients with high-risk stage II colon cancer after curative resection: a post hoc analysis of the JFMC46-1201 trial
International Journal of Colorectal Disease (2023)
-
Influence of Perioperative Anesthesia on Cancer Recurrence: from Basic Science to Clinical Practice
Current Oncology Reports (2023)
-
Anesthesia and cancer recurrence: an overview
Journal of Anesthesia, Analgesia and Critical Care (2022)