Abstract
Background:
The aim of this study was to evaluate the radiosensitising effect of gemcitabine, in terms of cell-cycle progression, induction of apoptosis, and to investigate the molecular events regulating apoptosis.
Methods:
Tumour cells were treated with gemcitabine, radiation, or the combination. 0–72 h after treatment, cells were collected for cell-cycle analysis and apoptosis determination. Caspase 8 and 9, Bid and tBid expression were determined by western blot. The mitochondrial membrane potential was determined using flow cytometry. An RT2 Profiler PCR Array for human apoptotic genes was performed after the combination or TRAIL treatment.
Results:
Gemcitabine and radiation resulted in an early S-phase block immediately after treatment, after which the cells moved synchronously through the cell cycle. When cell-cycle distribution returned to pre-treatment levels, an increased induction of apoptosis was observed with activation of caspase 8 and 9 and a reduction of the mitochondrial membrane potential. Gene expression after treatment with radiosensitising conditions was comparable with expression after the TRAIL treatment.
Conclusion:
A role for the cell-cycle perturbations and the induction of apoptosis could be attributed to the radiosensitising effect of gemcitabine. Apoptosis induction was comparable with the apoptotic pathway observed after the TRAIL treatment, that is the involvement of the extrinsic apoptosis pathway.
Similar content being viewed by others
Main
Killing of tumour cells by cytotoxic therapies, such as chemotherapy and γ-irradiation, is in some tumours predominantly mediated by triggering apoptosis (Brown and Wouters, 2001; Debatin and Krammer, 2004). This might occur either as a primary event induced by therapy or as a secondary event after lethal damage to the cell (Tannock and Lee, 2001). A family of cystein-dependent aspartate directed proteases, called caspases, is responsible for the proteolytic cleavage of cellular proteins leading to the characteristic apoptotic features. Currently, two pathways for activating caspases have been studied in detail, that is the mitochondrial ‘intrinsic’ pathway and the transmembrane ‘extrinsic’ pathway. Both pathways share the same effector caspases (caspase 3, 6, and 7).
The intrinsic pathway is under control of the Bcl-2 protein family. Permeabilisation of the outer mitochondrial membrane induces the leakage of proapoptotic molecules from the mitochondrial intermembrane space. In the cytosol, cytochrome c induces the oligomerisation of apoptosis protease activating factor 1 (Apaf-1) in the presence of ATP or dATP. Apoptosis protease activating factor 1 oligomers recruit procaspase 9 molecules in a complex called the ‘apoptosome’. The release of mature caspase 9 activates additional caspase 9 molecules as well as caspase 3 and 7. In turn, caspase 3 activates downstream caspase cascades. At the same time, the release of Smac/DIABLO and HtrA2/Omi neutralises the inhibitory effects of inhibitors of apoptosis proteins on caspase 3, 7, and 9.
Plasma membrane receptors for triggering external apoptosis signalling belong to the tumour necrosis factor (TNF)-receptor superfamily. The best-studied death receptor is Fas; binding of Fas ligand (FasL) leads to receptor trimerisation and recruitment of specific adaptor proteins. The Fas receptor contains a death domain (DD) in its cytoplasmatic region, which interacts with the adaptor protein, Fas-associating DD protein (FADD), forming a death receptor-induced signalling complex (DISC). Besides a DD, FADD contains a death effector domain (DED) and this recruits the DED-containing procaspase 8 into the DISC. Procaspase 8 will be proteolytically activated to the enzymatically active caspase 8, which in turn will activate downstream effector caspases. Other death receptors activate caspases in a similar manner. Depending on the cell type, activated caspase 8 induces apoptosis by two different signalling pathways. In type I cells, large amounts of active caspase 8 formed at the DISC induce direct activation of procaspase 3 independently of mitochondria. In type II cells, the presence of only very little DISC and small amounts of caspase 8 is insufficient to activate procaspase 3 directly and therefore amplification of the apoptotic signal through the mitochondrial apoptosis pathway is required. Instead, caspase 8 cleaves the ‘BH3-only protein’ Bid, generating an active fragment (tBid) that activates the mitochondrial death pathway (Green, 2000; Debatin and Krammer, 2004; Fulda and Debatin, 2006).
The nucleoside analogue gemcitabine (dFdC) has shown promising clinical effectiveness against a range of solid tumours. The cytotoxic effect of this agent is mediated by the induction of apoptotic cell death as shown by Huang and Plunkett (1995a). In addition, gemcitabine has shown both in laboratory and clinical studies to be a potent radiosensitiser (Pauwels et al, 2005a), but the exact mechanism of radiosensitisation remains as yet unknown. Interesting in that respect is the fact that gemcitabine-induced accumulation of cells in the S phase appears to be required for maximal radiosensitisation (Pauwels et al, 2005a; Shewach and Lawrence, 2007). Taking into account that the S phase is not reported to be the most radiosensitive phase of the cell cycle (Sinclair, 1972; Tang et al, 1997), the question remains whether the influence on the cell cycle indeed plays a role in radiosensitisation. Studies investigating the radiosensitising effect of gemcitabine hypothesised that the ability of cells to progress through the S phase after gemcitabine and radiation may be a key for radiosensitisation to occur (Ostruszka and Shewach, 2000; Mose et al, 2003). It may suggest that cells progressing beyond the S-phase block might accumulate proapoptotic signals, caused by both radiation and gemcitabine, resulting in increased cell death. It has also been hypothesised that radiosensitisation by gemcitabine is the result of lowering the threshold for radiation-induced apoptosis (Doyle et al, 2001).
Therefore, the aims of this study were to evaluate the cell-cycle progression and to study the induction of apoptosis, in two types of human tumour cells, after treatment with gemcitabine and/or radiotherapy. In addition, the molecular events that regulate apoptosis were explored. Understanding these events may provide important new opportunities for pathway-based rational therapy and for drug development.
Materials and methods
Cell lines
The cell lines used in this study were ECV304 (mutant (mt) p53), a human epidermoid bladder cancer cell line and H292 (wild-type (wt) p53), a human mucoepidermoid lung cancer cell line. H292 was cultured in RPMI-1640 medium, supplemented with L-glutamine, sodium pyruvate, and 10% fetal calf serum (Invitrogen, Merelbeke, Belgium). ECV304 was cultured in medium-199 supplemented with 10% fetal calf serum. Cultures were maintained in exponential growth in a humidified atmosphere at 37°C under 5% CO2/95% air. For subsequent experiments, cells were collected by trypsinisation, counted, and plated as specified below.
As we reported earlier, the IC50 value of 24 h treatment with gemcitabine was 3.05±0.49 for ECV304 cells and 7.99±0.77 for H292 cells (Pauwels et al, 2003a).
Cell survival after treatment with gemcitabine and radiation
Cells were plated in 48-well plates and treated as we described earlier (Pauwels et al, 2003a, 2003c, 2005b). Gemcitabine was added during 24 h, immediately followed by radiation or immediately after radiation (room temperature, 0–8 Gy, linear accelerator). After 7 days, the survival was determined by the sulforhodamine B (SRB) assay as described earlier (Pauwels et al, 2003a, 2003b). This method was comparable with the clonogenic assay taking in account some critical aspects (Pauwels et al, 2003b).
Cell cycle effect of gemcitabine and/or radiation
Cells were plated in 6-well plates as described earlier (Pauwels et al, 2003c). To ensure exponential growth during the experiments, seeding densities ranged from 0.3 to 0.5 × 105 cells per well depending of culture time after treatment. Cells were treated with 8 nM of gemcitabine for 24 h, or irradiated (4 Gy), or treated with 8 nM gemcitabine for 24 h immediately before 4 Gy radiation. Medium was replaced immediately after radiation, and the cell cycle was monitored for the following 72 h, by measuring cellular DNA content as published earlier (Pauwels et al, 2003c).
Determination of apoptosis by Annexin V staining, TUNEL assay, and caspase 3 activity assay
Cells were plated in 75 cm2 culture flasks to ensure exponential growth during the experiments. After plating and a 24 h recovery period, ECV304 and H292 cells were treated with IC25 (2 and 4 nM, respectively) or IC90 (8 and 18 nM, respectively) concentrations gemcitabine for 24 h, immediately before or after radiotherapy (2 and 6 Gy). Seventy-two hours later, both adherent and detached cells were collected.
Annexin V staining was performed using Annexin V-FITC apoptosis detection kit I (Becton-Dickinson Pharmingen, Erembodegem, Belgium) in accordance with the manufacturer's instructions. Briefly, cells were washed twice with cold PBS, counted and 1 × 106 cells were collected and resuspended in 1 ml binding buffer (10 mM HEPES/NaOH, 140 mM NaCl, 25 mM CaCl2). A measure of 100 μl of this solution was mixed with 5 μl Annexin V conjugated with fluorescein isothiocyanate (Annexin V-FITC) and 5 μl propidiumiodide (PI). The cells were gently vortexed and incubated for 15 min at room temperature. Then, 400 μl binding buffer was added. Analysis of green (Annexin V-FITC) and red (PI) fluorescence was performed in a FACScan flow cytometer (Becton-Dickinson).
To determine apoptosis by TUNEL assay, 2 × 106 cells were collected and evaluated using in situ cell death detection kit, Fluorescein (Roche, Vilvoorde, Belgium) in accordance with the manufacturer's instructions. Briefly, cells were washed twice with PBS/1%BSA at 4°C. Then, 1 ml fixation solution was added to the cell suspension (1% formaldehyde) for 30 min on ice, while shaking. Cells were centrifuged (5 min, 1200 r.p.m.) and washed once with PBS. Cells were permeabilised in 70% ethanol for at least 30 min at −20°C until colouring. Cells were washed twice with PBS and incubated in TUNEL reaction mixture (dUTP-FITC) for 60 min at 37°C. Cells were washed again twice with PBS and resuspended in 500 μl PBS containing 5 μl PI/RNase (500 μg ml−1 with 0.1% RNase). Samples were analysed in a FACScan flow cytometer, measuring green (dUTP-FITC incorporated in fragmented DNA) and red (PI binding to DNA) fluorescence of nuclei of individual cells.
Caspase 3 activity was determined using the colorimetric caspase 3 assay (Sigma Aldrich, Bornem, Belgium). This assay is based on the hydrolysis of the peptide substrate acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) by caspase 3, resulting in the release of pNA. pNA has a high absorbance at 405 nm (ɛnm=10.5). The concentration of pNA released from the substrate is calculated from a calibration curve prepared with defined pNA solutions; 107 control and treated cells were collected. Cells were washed with PBS and evaluated according to the manufacturer's instructions.
Mitochondrial transmembrane potential measurement
The mitochondrial membrane potential (Δψm) was flow cytometrically determined using a mitochondrial-sensitive probe: tetramethylrhodamine methylester (TMRM). It accumulates in the mitochondria and the transmembrane distribution of the dye is directly related to the membrane potential. Tetramethylrhodamine methylester does not accumulate in depolarised mitochondria. The extent of its uptake, measured as fluorescence intensity reflects Δψm.
Cells were plated in 75 cm2 culture flasks as described above. Seventy-two hours after treatment with gemcitabine (IC25 and IC90) and/or radiotherapy (2 and 6 Gy), both adherent and detached cells were collected. After washing in PBS, cells were incubated with 200 nM TMRM and 5 μl Annexin V-FITC for 15 min at 37°C and orange (TMRM) and green fluorescence were measured on a FACScan flow cytometer.
Western blot and immunodetection
Cells were plated in 75 cm2 culture flasks to ensure exponential growth during the experiments. After plating and a 24 h recovery period, cells were treated with gemcitabine alone (IC25 and IC90), radiation alone (2–6 Gy), or the combination. At different time points after radiation, both adherent and detached cells were collected by trypsinisation; 106 cells were washed with PBS and resuspended in 100 μl laemmli sample buffer (Bio-Rad, Nazareth, Belgium). Western blotting and immunodetection were performed as described earlier (Pauwels et al, 2005b). Primary antibodies were 1/100 anti-caspase 8 (Ab-3) (Calbiochem, Leuven, Belgium), 1/100 anti-caspase 9 (Ab-2) (Calbiochem), 1/1000 anti-Bid and truncated Bid (tBid) (Bioké, Leiden, The Netherlands). Secondary antibodies were 1/1000 Anti-rabbit IgG peroxidase conjugate and 1/1000 anti-mouse IgG horse-radish-peroxidase linked (Bioké).
Human apoptosis PCR array
A PCR array was used for gene expression analysis, taking advantage of real-time PCR performance, combined with the ability to detect the expression of many genes simultaneously.
Cells were plated in 75 cm2 culture flasks as described above. Seventy-two hours after treatment, total RNA samples were isolated from 4 × 106 control and treated cells (IC90–6 Gy) or cells treated with an inducer of the extrinsic pathway (200 ng ml−1 TRAIL, 6 h, Calbiochem), using RNeasy Mini Kit (Qiagen, Venlo, The Netherlands). TRAIL-induced apoptosis was first confirmed after Annexin V staining (data not shown). The cDNAs were reversed transcribed from RNA using ReactionReady First Strand cDNA Synthesis Kit (SABiosciences, Tebu-Bio, Boechout, Belgium). Comparison of the relative expression of 84 apoptosis-related genes was characterised by human apoptosis PCR array (SABiosciences) and the RT2real-time SYBR Green/Rox PCR Master mix (SABiosciences) on a 7000 real-time PCR System (Applied Biosystems, Lennik, Belgium). The array includes the TNF ligands and their receptors, members of the Bcl-2 family, caspases, IAP, TRAF, CARD, DD, DED, and CIDE family, as well as genes involved in the p53 and ATM pathways (Supplementary Figure 1).
Data analysis
Survival rates were calculated by mean OD (optical density) of treated cells/mean OD of control cells × 100%. The radiation survival curves were fitted according to the linear quadratic model: survival=exp(−αD–βD2), using Winnonlin (Pharsight, Mountain View, CA, USA). The radiosensitising effect was represented by the dose enhancement factor (DEF): ID50(−dFdC)/ID50(+dFdC). For the determination of synergism, the combination index (CI) was calculated by the Chou–Talalay equation (Pauwels et al, 2003a), using CalcuSyn (Biosoft, Cambridge, UK). A CI value between 0.9 and 1.1 indicates only additivity. Moderate synergism is depicted by CI values between 0.7 and 0.9, synergism by CI values below 0.7.
Flow cytometric data were analysed using Cell Quest (Becton-Dickinson) software.
Unless otherwise indicated, all data are presented as the mean±s.d. All experiments were performed at least three times. A two-sample t-test and two-way ANOVA were used to determine statistical significance (P<0.05). Two-way ANOVA was used to study the impact of the concentration of gemcitabine, doses radiotherapy, and treatment schedule on the outcome parameter (cell survival). Post hoc comparisons revealed which groups differed significantly from one another. A correlation coefficient was calculated between apoptosis induction and cell survival.
To analyse the data of the PCR array, a centroid-mediated classification algorithm was applied. Therefore, two centroids, representing the average gene expression pattern of the apoptosis-related genes with or without treatment with TRAIL, were calculated for H292 and ECV304 cells separately. Thereafter, the agreement between the apoptosis-related gene expression pattern of control cells or cells treated with gemcitabine and radiotherapy and the apoptosis-related gene expression pattern with or without treatment with TRAIL were quantified for each cell line. Therefore, Pearson correlation coefficients between the apoptosis-related expression pattern of each centroid (TRAIL-treated and TRAIL-untreated) and the apoptosis-related expression patterns of the cell lines (control or gemcitabine and radiation-treated cells) were calculated. Then, for each centroid separately, the correlation coefficients were compared between the control and the treatment conditions.
Next, P-values were calculated to identify individual genes with gene expression differences between the untreated and treated experiments for each cell line separately. Using the global test (Goeman et al, 2004), geneplots of the differentially expressed genes were generated to investigate the relative overexpression of each gene present in the gene list of differentially expressed genes. In addition, to account for the false discovery rate, the global test was used to calculate a Z-score for each of the differentially expressed genes between treated and untreated experiments. The Z-score represents the difference between the observed and expected (calculated by random class label permutations) gene expression differences between treated and untreated cells, normalised to the standard deviation of the distribution of expected gene expression differences.
Results
Cell survival after treatment with gemcitabine and radiation
A clear concentration-dependent radiosensitising effect of gemcitabine was observed in ECV304 and H292 cells, when cells were treated during 24 h with gemcitabine before radiotherapy (Figure 1). This radiosensitising effect was not observed when cells were treated with gemcitabine during 24 h immediately after irradiation. When gemcitabine treatment preceded radiation, DEFs ranged from 1.39 to 3.05 (CI values 1.05–0.65) in ECV304 cells and from 1.20 to 2.67 (CI values 1.21–0.76) in H292 cells (Pauwels et al, 2003a). When gemcitabine followed radiotherapy DEFs ranged from 0.98 to 1.02 and from 1.12 to 1.19 for ECV304 and H292 cells, respectively, with CI values above 0.827 for ECV304 and above 1.071 for H292 cells. Statistical analysis using two-way ANOVA revealed that cell survival was significantly influenced by the concentration of gemcitabine, the dose of radiation, and the treatment schedule (gemcitabine before or after radiotherapy) in both cell lines. Post hoc analysis showed in ECV304 significant differences among all radiation doses and also among 0, 1, and 2 nM gemcitabine. No significant difference was observed between 2 and 4 nM gemcitabine (P=0.865). In H292 cells, significant differences were observed among all radiation doses and between 0 and 4 nM gemcitabine. No significant differences were observed among 4, 6, and 8 nM gemcitabine (P>0.724).
Cell cycle effect after the combination gemcitabine and radiation
In Figure 2, the distribution of ECV304 and H292 cells over the cell cycle phases at different time points is shown. Without treatment, the distribution of cells over the different phases is very similar over time. Treatment with gemcitabine (8 nM) alone resulted in an early S-phase block immediately after treatment, as we reported earlier (Pauwels et al, 2003c) and 8 h later, the amount of S-phase cells increased and 48 h after treatment the cell cycle distribution returned to pre-treatment levels. In ECV304 cells, radiation (4 Gy) caused a G2/M block, which was maximal 16 h after radiotherapy. In H292 cells, radiotherapy resulted in a G2/M block, which was also maximal 16 h after radiotherapy, whereas the amount of G1 cells remained roughly constant at the expense of S-phase cells. 48 h after radiation, the cell cycle distribution returned to pre-treatment levels. Treatment with the combination of gemcitabine and radiotherapy resulted in an early S-phase block immediately after treatment, both in ECV304 and in H292 cells. This block was followed by a synchronous movement of the cells to the G2/M phase. The G2/M block was maximal 24 h after treatment, being 8 h later than after radiotherapy alone. The cell cycle distribution returned to pre-treatment levels 72 h after combination treatment.
Analysis of apoptosis induction after treatment with gemcitabine and/or radiotherapy
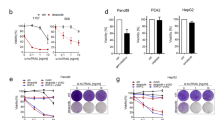
The amount of apoptotic cells was determined using Annexin V staining and caspase 3 activity assay. For ECV304 cells, apoptosis induction was also evaluated by TUNEL assay. In Table 1, the amount of Annexin V-positive cells is summarised. For both ECV304 and H292 cells, the amount of early apoptotic cells increased with the combination gemcitabine and radiation. More apoptotic cells were observed with a higher concentration gemcitabine or higher radiation dose. Similar results were observed with TUNEL assay for ECV304 cells (Figure 3A). In fact, treatment with 8 nM of gemcitabine and 6 Gy radiation resulted in more than 50% TUNEL-stained cells 72 h after treatment.
(A) TUNEL-positive ECV304 cells 72 h after treatment with gemcitabine, radiotherapy, or the combination of gemcitabine and radiotherapy. Dot plot from a representative experiment (R2=normal cell population, R3=apoptotic cell population). (B) Caspase 3 activity of ECV304 and H292 cells 72 h after treatment with gemcitabine, radiotherapy, or the combination of gemcitabine and radiotherapy. *P<0.05 vs 0 nM–0 Gy, †P<0.05 vs 0 nM–6 Gy, §P<0.05 vs 8 nM–0 Gy. (C) Caspase 8, caspase 9, Bid and tBid expression of H292 cells 72 h after gemcitabine (IC25, IC90) and/or radiotherapy. Similar results were observed with ECV304 cells. (D) Annexin V-positive cells with reduced TMRM fluorescence (loss of Δψm) and TMRM-positive cells after treatment with gemcitabine, radiotherapy, or the combination. *P<0.05 vs 0 nM–0 Gy, †P<0.05 vs 2 nM–0 Gy, §P<0.05 vs 0 nM–2 Gy, ∣∣P<0.05 vs 0 nM–6 Gy, **P<0.05 vs 8 nM–0 Gy, ††P<18 nM–0 Gy.
Using the caspase 3 activity assay, these results were confirmed. Caspase 3 activity increased with the combination of gemcitabine and radiotherapy (Figure 3B).
Although apoptosis was determined when cells were treated with gemcitabine immediately after radiation, Annexin V staining was less pronounced than when gemcitabine treatment preceded radiation (Table 1). In H292 cells, this is significantly different.
The amount of apoptosis of ECV304 and H292 cells was negatively correlated to the cell survival observed with the SRB test for the IC25 values of gemcitabine (the correlation coefficient was −0.8 and −0.9 for ECV304 and H292 cells, respectively, when apoptosis was determined with Annexin V staining). This means that there is a positive correlation between apoptosis induction and the amount of cell kill using lower concentrations of gemcitabine. The correlation coefficient for the IC90 values could not be calculated because this concentration was not used in the SRB test because of high toxicity.
Immunodetection of caspase 8, caspase 9, Bid and tBid after treatment with gemcitabine and radiotherapy
To investigate the apoptotic pathway under radiosensitising conditions, the expression of caspase 8 and 9, initiator caspases of, respectively, the receptor and mitochondrial-mediated pathway were investigated at the moment apoptosis was observed, that is 72 h after combination treatment.
Gemcitabine alone induced the activation of caspase 8 in H292 and ECV304 cells. In H292, both IC25 and IC90 gemcitabine concentrations resulted in caspase 8 cleaving. No activation of caspase 9 could be shown after incubation with gemcitabine alone. A similar effect was observed after irradiation alone (data not shown). When cells were treated with the combination of gemcitabine and radiotherapy, cleaving products of both caspase 8 and 9 were observed. This could mean that both the extrinsic and intrinsic apoptotic pathway were involved in the apoptotic cell death. Therefore, Bid and tBid expression were determined. However, Bid expression was quite variable and did not show any relationship with treatment. No tBid could be shown (Figure 3C).
Measurement of the transmembrane mitochondrial potential
In Figure 3D, TMRM-positive cells and Annexin-V-positive cells with a reduction in TMRM fluorescence (loss of Δψm) are shown. Treatment with gemcitabine, radiotherapy but especially with the combination of both did increase the amount of cells with a loss of mitochondrial potential. Thus, induction of apoptosis under radiosensitising conditions is accompanied by decrease in Δψm.
Human apoptosis PCR array
In Figure 4A, the correlation coefficient of control and treated cells with the centroids (no TRAIL or TRAIL) are shown. When the correlation coefficient of treated cells (IC90-6 Gy) and the centroid without TRAIL are compared with the correlation coefficients of the treated cells (IC90-6 Gy) and the centroid with TRAIL, the latter correlation coefficients are significantly elevated. When the curve slopes were determined and compared for each cell line separately, there was a significant difference for ECV304 (P=0.002), whereas there was only a trend to difference for H292 cells (P=0.095). This means that the gene expression pattern of ECV304 after treatment with the combination of gemcitabine and irradiation was comparable with the gene expression pattern of cells treated with TRAIL. In H292 cells, gene expression pattern using radiosensitising conditions was also comparable with the pattern after treatment with TRAIL, however, to a lesser extent then in ECV304 cells.
(A) Standard correlation coefficient of ΔΔCt values of ECV304 and H292 cells (0 nM–0 Gy and IC90-6 Gy), compared with the centroid of ECV304 and H292 cells (no TRAIL or TRAIL). Results of three different experiments and mean of the experiments are shown. (B) Global test for group of genes. Individual genes with significant gene expression differences between the untreated (0 nM–0 Gy) and treated (IC90-6 Gy) experiments are shown (P<0.05) for ECV304 and H292 cells.
In Figure 4B, the individual genes with significant gene expression differences between the untreated (0 nM–0 Gy) and treated (IC90-6 Gy) experiments are shown (P<0.05). In ECV304 cells, expression of caspase 6, BID, BIRC3, GADD45A, and CARD8 was more pronounced in samples of treated cells. In H292 cells, GADD45A, caspase 8, caspase 4, caspase 5, TNFRSF9, PYCARD, BCL2L1, and Fas showed a higher expression in cells after treatment with gemcitabine and radiotherapy. All these genes are pro-apoptotic genes, except BIRC3 for ECV304 cells and BCL2L1 for H292 cells.
Discussion
In this study, the role of apoptosis in the radiosensitising effect of gemcitabine was investigated thoroughly. An increased induction of apoptosis was observed using radiosensitising conditions as a result of activation of the extrinsic or receptor-mediated apoptosis pathway. This is the first study investigating the apoptosis pathway after treatment of tumour cells with radiosensitising conditions of gemcitabine.
Apoptosis has shown to be a significant mode of cell death after tumour treatment and may play a significant role in drug and radiation enhancement (Tolis et al, 1999; Ostruszka and Shewach, 2000; Fukuoka et al, 2001; Lawrence et al, 2001; Liu et al, 2001). However, this might be the case only in some tumour types and might occur either as a primary event induced by therapy or as a secondary event after lethal damage to the cell (Brown and Wouters, 2001; Tannock and Lee, 2001) Increased induction of apoptosis is observed with the radiosensitising effect of taxanes (Creane et al, 1999), vinorelbine (Fukuoka et al, 2001; Zhang et al, 2004), campthotecin (Rich, 2003), and oxaliplatin (Hermann et al, 2008). However, clinical data do not provide definitive evidence for the role of apoptosis in the response to cancer therapy, and in fact contradicting reports were published, also concern the apoptotic pathway (Brown and Wilson, 2003). Although some studies assign a key role for the mitochondrial pathway in drug-induced apoptosis and the death-receptor pathway may amplify this, other studies suggest that mitochondria may act as amplifiers, but not initiators of cell death (Debatin and Krammer, 2004; Fulda and Debatin, 2006). However, further insight into the complex signalling network activated in response to anticancer therapy is necessary to see to what extent the current knowledge can be exploited for the design of new cancer therapies (Fulda and Debatin, 2006).
As showed earlier, a clear radiosensitising effect has been observed when gemcitabine treatment precedes irradiation (Shewach and Lawrence, 1996; Pauwels et al, 2005a). It has also been shown that the cell cycle effect of gemcitabine, being a block in the early S-phase, is correlated with the radiosensitising effect (Pauwels et al, 2005a). In this study the cell cycle progression after treatment with the combination of gemcitabine and radiotherapy initially resulted in an early S-phase block, after which the cells progressed through the cell cycle. Seventy-two hours after treatment, the cell cycle distribution returned to pre-treatment levels. We observed that the amount of cells in culture had decreased at that time and because the S-phase is not the most radiosensitive phase (Sinclair, 1972; Tang et al, 1997), we hypothesised that the blocked S-phase cells underwent apoptosis. An increased induction of apoptosis was observed using radiosensitising conditions as determined by Annexin V staining, caspase 3 activity assay, and TUNEL assay. When gemcitabine followed radiation, less apoptotic cells were observed. This schedule did not result in a radiosensitising effect either. It seemed that apoptosis was induced after completion of S and G2/M phase and that the cell cycle perturbations followed by the induction of apoptosis play an important role in the radiosensitising effect. Wachters et al have shown that gemcitabine causes radiosensitisation by specific interference with homologous recombination-mediated repair and that homologues recombination repair is preferentially used in late S and G2 phases of the cell cycle (Wachters et al, 2003), supporting our observations that apoptosis was induced after completion of these cell cycle phases. Gemcitabine is known to induce apoptosis by itself when used in cytotoxic concentrations (Huang and Plunkett, 1995b; Huang et al, 1997). However, additional involvement of apoptosis in the radiosensitising effect of gemcitabine has been reported also (Doyle et al, 2001; Lawrence et al, 2001; Zhang et al, 2004) and our findings are consistent with these reports. Lawrence et al concluded indeed that apoptosis plays an important role in gemcitabine-mediated radiosensitisation, but considered this not to be the sole mechanism responsible for radiosensitisation, based on the findings that the largest effect was observed in the apoptotic-prone HT-29 cells and only modest apoptosis in UMSCC-6 and A549 cells (Lawrence et al, 2001). Lawrence et al and Doyle et al showed that it is a misconception that a defect in apoptosis should restore a treatment-responsive state to the cell. They showed that cell death occurs according to the mode of death intrinsic to the cell line (Doyle et al, 2001; Lawrence et al, 2001). In our study, a correlation was found between the increase in apoptotic cells and the cell survival obtained with the SRB test with the IC25 concentrations of gemcitabine. The lesser response of H292 cells using the SRB test in Figure 1 in correlation with apoptosis induction may be explained by the different determination times; that is apoptosis was determined 72 h after treatment, whereas cell survival is determined after 7 days.
The study of Lawrence et al only investigated the terminal parts of the apoptotic pathway (caspase 3 activation and morphological changes). Apoptosis can results from the activation of several upstream pathways including TNF-receptor stimulation and mitochondrial release of cytochrome c (Green, 1998). The second part of our study was to determine which of these pathways were activated during radiosensitisation, because the possibility remained that selective potentiation of the active pathway could increase the effectiveness of gemcitabine as a radiation sensitiser (Lawrence et al, 2001).
Using radiosensitising conditions, both active caspase 8 and 9 could be observed by western blot. Therefore, the mitochondrial membrane potential was measured. These experiments confirmed the involvement of the mitochondria after treatment of the cells with the combination of gemcitabine and radiotherapy and because of these results, it seemed that gemcitabine and radiotherapy initially activated the extrinsic apoptosis pathway, but that amplification of this apoptosis signal by the mitochondrial pathway seemed required. Therefore, the activation of Bid protein was determined. Bid provides the only known connection between the extrinsic and the intrinsic pathways (Kuribayashi and El-Deiry, 2008). Unfortunately, the activated form tBid could not be observed. To further investigate in depth the pathway induced by radiosensitising conditions of gemcitabine, an apoptosis PCR array was performed. We could show that gene expression after treatment with gemcitabine and radiation was comparable with gene expression after treatment with TRAIL. This was significant in ECV304 cells. The similarity was less for H292 cells. TRAIL is a known inducer of the extrinsic apoptosis pathway (Thorburn, 2007). This could mean that in ECV304 cells (mt p53), the increased induction of apoptosis after the combination of gemcitabine and radiotherapy is a result of the activation of the extrinsic apoptosis pathway, whereas in H292 cells, which are wt p53 cells, this pathway is very likely activated, but another apoptosis pathway must be involved also. Tolis et al showed that gemcitabine exerts its antitumour effect by the apoptotic machinery both in wt and mt p53 cells, indicating the presence of p53 independent pathways (Tolis et al, 1999). The difference between the two cell lines was also observed when we looked to the genes that were significantly higher expressed in treated cells (Figure 4B). In H292 cells, GADD45A, caspase 8, and Fas were expressed more pronounced after treatment with gemcitabine and radiotherapy, and it is known that these genes are transcriptionally regulated by p53. GADD45A can also be induced by p53 independent mechanisms after DNA damage (source: http://www.ncbi.nlm.nih.gov/pubmed/, 2008) as observed in ECV304 cells. Using the PCR array, we observed an increase in expression of Bid in ECV304 cells after using radiosensitising conditions, whereas we could not observe this using a western blot. Most of the upregulated genes are involved in a caspase-dependent programmed cell death.
Studies investigating the mechanism of apoptosis induction after gemcitabine treatment alone have been published with variable outcome. Most studies showed the involvement of the death receptor pathway. Pace et al and Gazzaniga et al showed that gemcitabine controls tumour progression by an increased sensitivity of tumour cells to Fas-dependent apoptosis (Pace et al, 2000; Gazzaniga et al, 2007). However, Ferreira et al showed caspase 8 activation independently from Fas/fasL signalling (Ferreira et al, 2000b) and concluded that caspase 8 forms the apical and mitochondria-dependent step that subsequently activates downstream caspases (Ferreira et al, 2000a). In multiple myeloma cell lines, it was shown that gemcitabine induced apoptosis with caspase 3, 8, 9, and PARP cleavage, indicating that several mechanisms of action, including death receptor pathway and mitochondrial damage, are involved (Nabhan et al, 2002). In addition, in pancreatic cells, caspase 8 was activated before the breakdown of the mitochondrial transmembrane potential (Christgen et al, 2005). However, though these studies are in line with our observations, other studies found the involvement of other apoptotic signalling pathways (Chang et al, 2004; Habiro et al, 2004; Schniewind et al, 2004; Kurdow et al, 2005; Okamoto et al, 2007).
In summary, we could grant a role for the cell cycle perturbations and for the induction and mechanism of apoptosis in the radiosensitising effect of gemcitabine. It seems that the combination of gemcitabine and radiotherapy activates the extrinsic apoptosis pathway. The involvement of caspase 9, the intrinsic pathway, is a secondary event, possibly resulting in an enhancement of apoptosis. Apoptosis induction was very similar to the apoptotic pathway after TRAIL treatment. Because gemcitabine can enhance TRAIL-induced apoptosis (Seol et al, 2007), the use of gemcitabine with TRAIL combined with radiation could be promising. However, apoptosis does not represent the sole killing mechanism by which cancers are eradicated, and other modes of cell death, that is necrosis, autophagy, and mitotic catastrophe, are some forms of cell death that cannot be easily classified at present, and have to be considered also (Debatin and Krammer, 2004; Fulda and Debatin, 2006). The primary mechanisms behind gemcitabines radiosensitisation do not necessarily invoke the apoptotic machinery. Impairment of double strand break repair through the homologous recombination pathway (Wachters et al, 2003) or futile mismatch repair cycles at replication forks (because of precursor imbalance), or both may provoke apoptosis or result in mitotic catastrophe. Different forms of cell death should be considered when addressing the question of radiation-induced cell damage or cell survival after irradiation (Abend, 2003).
This study reveals important new insights into the mechanism of radiosensitisation, and can form a solid basis for further studies on the role of apoptosis signalling molecules in clinical samples using DNA or proteomic arrays. These studies are warranted to assess the impact of these molecular parameters on clinical outcome.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abend M (2003) Reasons to reconsider the significance of apoptosis for cancer therapy. Int J Radiat Biol 79: 927–941
Brown JM, Wilson G (2003) Apoptosis genes and resistance to cancer therapy: what does the experimental and clinical data tell us? Cancer Biol Ther 2: 477–490
Brown JM, Wouters BG (2001) Apoptosis: mediator or mode of cell killing by anticancer agents? Drug Resist Updat 4: 135–136
Chang G, Hsu S, Tsai J, Wu W, Chen C, Sheu G (2004) Extracellular signal-regulated kinase activation and Bcl-2 downregulation mediate apoptosis after gemcitabine treatment partly via a p53-independent pathway. Eur J Pharmacol 502: 169–183
Christgen M, Schniewind B, Jueschke A, Ungefroren H, Kalthoff H (2005) Gemcitabine-mediated apoptosis is associated with increased CD95 surface expression but is not inhibited by DN-FADD in Colo357 pancreatic cancer cells. Cancer Lett 227: 193–200
Creane M, Seymour CB, Colucci S, Mothersill C (1999) Radiobiological effects of docetaxel (Taxotere): a potential radiation sensitizer. Int J Radiat Biol 75: 731–737
Debatin KM, Krammer PH (2004) Death receptors in chemotherapy and cancer. Oncogene 23: 2950–2966
Doyle TH, Mornex F, McKenna G (2001) The clinical implications of gemcitabine radiosensitization. Clin Cancer Res 7: 226–228
Ferreira CG, Span SW, Peters GJ, Kruyt FAE, Giaccone G (2000a) Chemotherapy triggers apoptosis in a caspase-8-dependent and mitochondria-controlled manner in the non-small cell lung cancer cell line NCI-H460. Cancer Res 60: 7133–7141
Ferreira CG, Tolis C, Span SW, Peters GJ, van Lopik T, Kummer AJ, Pinedo HM, Giaccone G (2000b) Drug-induced apoptosis in lung cancer cells is not mediated by the Fas/FasL (CD95/APO1) signaling pathway. Clin Cancer Res 6: 203–212
Fukuoka K, Arioka H, Iwamoto Y, Fukumoto H, Kurokawa H, Ishida T, Tomonari A, Suzuki T, Usuda J, Kanzawa F, Saijo N, Nishio K (2001) Mechanism of the radiosensitization induced by vinorelbine in human non-small cell lung cancer cells. Lung Cancer 34: 451–460
Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25: 4798–4811
Gazzaniga P, Silvestri I, Gradilone A, Scarpa S, Morrone S, Gandini O, Gianni W, Frati L, Agliano AM (2007) Gemcitabine-induced apoptosis in 5637 cell line: an in-vitro model for high-risk superficial bladder cancer. Anticancer Drugs 18: 179–185
Goeman JJ, van de Geer SA, de KF, van Houwelingen HC (2004) A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 20: 93–99
Green D (1998) Apoptotic pathways: the roads to ruin. Cell 94: 695–698
Green D (2000) Apoptotic pathways: paper wraps stone blunts scissors. Cell 102: 1–4
Habiro A, Tanno S, Koizumi K, Izawa T, Nakano Y, Osanai M, Mizukami Y, Okumura T, Kohgo Y (2004) Involvement of p38 mitogen-activated protein kinase in gemcitabine-induced apoptosis in human pancreatic cancer cells. Biochem Biophys Res Commun 316: 71–77
Hermann RM, Rave-Frank M, Pradier O (2008) Combining radiation with oxaliplatin: a review of experimental results. Cancer Radiother 12: 61–67
Huang P, Ballal K, Plunkett W (1997) Biochemical characterization of the protein activity responsible for high molecular weight DNA fragmentation during drug-induced apoptosis. Cancer Res 57: 3407–3414
Huang P, Plunkett W (1995a) Fludarabine- and gemcitabine-induced apoptosis: incorporation of analogs into DNA is a critical event. Cancer Chemother Pharmacol 36: 181–188
Huang P, Plunkett W (1995b) Induction of apoptosis by gemcitabine. Semin Oncol 22: 19–25
Kurdow R, Schniewind B, Zoefelt S, Boenicke L, Boehle A, Dohrmann P, Kalthoff H (2005) Apoptosis by gemcitabine in non-small cell lung cancer cell line KNS62 is induced downstream of caspase 8 and is profoundly blocked by Bcl-xL over-expression. Langenbecks Arch Surg 390: 243–248
Kuribayashi K, El-Deiry WS (2008) Regulation of programmed cell death by the p53 pathway. Adv Exp Med Biol 615: 201–221
Lawrence TS, Davis MA, Hough A, Rehemtulla A (2001) The role of apoptosis in 2 ‘,2 ‘-difluoro-2 ‘-deoxycytidine (gemcitabine)-mediated radiosensitization. Clin Cancer Res 7: 314–319
Liu XM, Wang LG, Kreis W, Budman DR, Adams LM (2001) Differential effect of vinorelbine versus paclitaxel on ERK2 kinase activity during apoptosis in MCF-7 cells. Br J Cancer 85: 1403–1411
Mose S, Class R, Weber HW, Rahn A, Brady LW, Bottcher HD (2003) Radiation enhancement by gemcitabine-mediated cell cycle modulations. Am J Clin Oncol 26: 60–69
Nabhan C, Gajria D, Krett NL, Gandhi V, Ghias K, Rosen ST (2002) Caspase activation is required for gemcitabine activity in multiple myeloma cell lines. Mol Cancer Ther 1: 1221–1227
Okamoto K, Ocker M, Neureiter D, Dietze O, Zopf S, Hahn EG, Herold C (2007) bcl-2-specific siRNAs restore gemcitabine sensitivity in human pancreatic cancer cells. J Cell Mol Med 11: 349–361
Ostruszka LJ, Shewach DS (2000) The role of cell cycle progression in radiosensitization by 2 ‘,2 ‘-difluoro-2 ‘-deoxycytidine. Cancer Res 60: 6080–6088
Pace E, Melis M, Siena L, Bucchieri F, Vignola AM, Profita M, Gjomarkaj M, Bonsignore G (2000) Effects of gemcitabine on cell proliferation and apoptosis in non-small-cell lung cancer (NSCLC) cell lines. Cancer Chemother Pharmacol 46: 467–476
Pauwels B, Korst A, Lardon F, Vermorken J (2005a) Combined modality therapy of gemcitabine and radiation. Oncologist 10: 34–51
Pauwels B, Korst AEC, Andriessen V, Baay MFD, Pattyn GGO, Lambrechts HAJ, De Pooter CMJ, Lardon F, Vermorken JB (2005b) Unravelling the mechanism of radiosensitisation by gemcitabine: the role of p53. Radiat Res 164: 642–650
Pauwels B, Korst AEC, De Pooter CMJ, Lambrechts HAJ, Pattyn GGO, Lardon F, Vermorken JB (2003a) The radiosensitising effect of gemcitabine and the influence of the rescue agent amifostine in vitro. Eur J Cancer 39: 838–846
Pauwels B, Korst AEC, De Pooter CMJ, Pattyn GGO, Lambrechts HAJ, Baay MFD, Lardon F, Vermorken JB (2003b) Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother Pharmacol 51: 221–226
Pauwels B, Korst AEC, Pattyn GGO, Lambrechts HAJ, Van Bockstaele DR, Vermeulen K, Lenjou M, De Pooter CMJ, Vermorken JB, Lardon F (2003c) Cell cycle effect of gemcitabine and its role in the radiosensitizing mechanism in vitro. Int J Radiat Oncol Biol Phys 57: 1075–1083
Rich TA (2003) Camptothecin radiation sensitization. In Chemoradiation in Cancer Therapy Choy H (ed), pp 93–105. Humana Press: Totowa
Schniewind B, Christgen M, Kurdow R, Haye S, Kremer B, Kalthoff H, Ungefroren H (2004) Resistance of pancreatic cancer to gemcitabine treatment is dependent on mitochondria-mediated apoptosis. Int J Cancer 109: 182–188
Seol JW, Chaudhari AA, Lee YJ, Kang HS, Kim IS, Kim NS, Park JH, Kim TH, Seol DW, Park SY (2007) Regulation of DR-5 protein and mitochondrial transmembrane potential by gemcitabine, a possible mechanism of gemcitabine-enhanced TRAIL-induced apoptosis. Oncol Rep 18: 523–529
Shewach DS, Lawrence TS (1996) Radiosensitization of human solid tumor cell lines with gemcitabine. Semin Oncol 23: 65–71
Shewach DS, Lawrence TS (2007) Antimetabolite radiosensitizers. J Clin Oncol 25: 4043–4050
Sinclair WK (1972) Sensitivity to mitotic delay and stage in the cycle. Curr Top Radiat Res 7: 323–327
Tang HY, Davis MA, Strickfaden SM, Maybaum J, Lawrence TS (1997) Influence of cell cycle phase on radiation-induced toxicity and DNA damage in human colon cancer (HT-29) and chinese hamster ovary cells. Radiat Res 138: S109–S112
Tannock IF, Lee C (2001) Evidence against apoptosis as a major mechanism for reproductive cell death following treatment of cell lines with anti-cancer drugs. Br J Cancer 84: 100–105
Thorburn A (2007) Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway signaling. J Thorac Oncol 2: 461–465
Tolis C, Peters GJ, Ferreira CG, Pinedo HM, Giaccone G (1999) Cell cycle disturbances and apoptosis induced by topotecan and gemcitabine on human lung cancer cell lines. Eur J Cancer 35: 796–807
Wachters FM, van Putten JWG, Maring JG, Zdzienicka MZ, Groen HJM, Kampinga HH (2003) Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys 57: 553–562
Zhang M, Boyer M, Rivory L, Hong A, Clarke S, Stevens G, Fife K (2004) Radiosensitization of vinorelbine and gemcitabine in NCI-H460 non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys 58: 353–360
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Pauwels, B., Vermorken, J., Wouters, A. et al. The role of apoptotic cell death in the radiosensitising effect of gemcitabine. Br J Cancer 101, 628–636 (2009). https://doi.org/10.1038/sj.bjc.6605145
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605145
Keywords
This article is cited by
-
The radiosensitising effect of gemcitabine and its main metabolite dFdU under low oxygen conditions is in vitro not dependent on functional HIF-1 protein
BMC Cancer (2014)
-
Difluorodeoxyuridine plasma concentrations after low-dose gemcitabine during chemoradiation in head and neck cancer patients
Cancer Chemotherapy and Pharmacology (2011)
-
In vitro study on the schedule-dependency of the interaction between pemetrexed, gemcitabine and irradiation in non-small cell lung cancer and head and neck cancer cells
BMC Cancer (2010)
-
Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway
Medical Oncology (2010)