Abstract
In a Japanese study, cyclin-dependent kinase (CDK) based risk determined by CDK 1 and 2 activities was associated with risk of distance recurrence in early breast cancer patients. The aim of our study was to validate this risk categorization in European early breast cancer patients. We retrospectively analyzed frozen breast cancer specimens of 352 Dutch patients with histologically confirmed primary invasive early breast cancer. CDK-based risk was determined in tumour tissues by calculating a risk score (RS) according to kinases activity and protein mass concentration assay without the knowledge of outcome. Determination of CDK-based risk was feasible in 184 out of 352 (52%) tumours. Median follow-up of these patients was 15 years. In patients not receiving systemic treatment, the proportions of risk categories were 44% low, 16% intermediate, and 40% high CDK-based risk. These groups remained significant after univariate and multivariate Cox-regression analysis. Factors associated with a shorter distant recurrence-free period were positive lymph nodes, mastectomy with radiotherapy, and high CDK-based risk. There was no significant correlation with overall survival (OS). CDK-based risk is a prognostic marker of distance recurrence of patients with early breast cancer. More validation would be warranted to use of CDK-based risk into clinical practice.
Similar content being viewed by others
Main
Breast cancer is the most commonly occurring cancer and the leading cause of cancer death in women in the Western world (Parkin et al, 2005). In addition to local therapy, systemic treatment improves disease-free and overall survival (OS) in patients with early breast cancer (Early Breast Cancer Trialists' Collaborative Group, 2005). Based on traditional prognostic markers, such as age, tumour grade/size and nodal status, patients are classified into different risk groups to determine who will receive systemic treatment (Goldhirsch et al, 2007). However, breast cancer can recur in low-risk patients not receiving systemic treatment, resulting in a poor clinical outcome. This indicates that these conventional prognostic markers are not yet optimal for risk assessment. Although several new tumour-related biological parameters are investigated, none of these has been introduced in standard clinical practice so far (Annecke et al, 2008; Cardoso et al, 2008; Sparano and Paik, 2008).
One relevant characteristic of tumours is their aggressiveness in proliferation, which is evaluated by such biological indicators as 3H-thymidine uptake, DNA-analysis, mitotic activity index (MAI) and Ki-67 expression. These approaches are not highly useful in clinical practice, because of technical and performance instabilities. It was shown that overexpression of cyclins, which bind and activate cyclin-dependent kinases (CDKs), as well as inactivation of CDK inhibitors such as p21WAF1 and p27Kip1, which inhibit CDK activities, correlates with prognosis in a variety of malignancies (Gillett et al, 1994; Gramlich et al, 1994; Gansauge et al, 1997; Sutter et al, 1997; van Diest et al, 1997; Nakashima et al, 2000; Sjostrom et al, 2000; Takano et al, 2000; Ishihara et al, 2005). Therefore, direct measurement of CDK activity could provide more reliable clinical information about the prognosis than used molecular pathological parameters.
On the basis of these considerations, an assay system was developed that can directly measure the activity and expression of CDK1 and CDK2 in a routine laboratory test (SA; the kinases activity divided by the protein mass concentration) was established (Ishihara et al, 2005). The clinical performance of the system was first evaluated in a Japanese retrospective study in 284 early breast cancer patients with a median follow-up of almost 5 years (Kim et al, 2008). It was found that CDK-based risk derived from the SA of CDK1 and CDK2 was associated with risk of relapse. However, the procedure to determine this CDK-based risk is complicated and intuitive. Therefore, the data of the last Japanese study was re-evaluated to define a risk score (RS), which quantitatively indicates the risk for recurrence.
The aim of our study was to validate the prognostic value of the modified CDK-based risk recurrence model in a European patient population and to examine if CDK-based risk is correlated with established prognostic factors. In turn, these results may be used to enable better risk identification for early breast cancer patients as the basis for better risk adapted individualised adjuvant systemic treatment decisions.
Materials and methods
Patients
A consecutive series of patients with histologically confirmed invasive early breast cancer that received primary surgical resection in the Leiden University Medical Centre between 1985 and 1996 was used. Patients with an earlier history of cancer (other than basal cell carcinoma or cervical in situ carcinoma), bilateral tumours or a secondary tumour other than breast cancer, were excluded. The following data were available: age at diagnosis, histological type, TNM stage, local and systemic therapy, locoregional and distant recurrence, second primaries, and OS. All tumours were regraded by one pathologist (VS). Approval was obtained from the LUMC Medical Ethics Committee.
Sample preparation
Tumour tissue was dissected from the surgical resection, immediately embedded in optimal cutting temperature (OCT) compound, and stored at −80°C. Ten to 20 sections of 100 μm thickness were cut from the embedded tissue with a cryostat and subjected to CDK analysis as described below. To analyze the influence of OCT contamination to the assay system, the OCT content at the surface of the cryosection was recorded as a percentage.
Determination of CDK-specific activities
The system to measure the CDK SA is named C2P® (Sysmex, Kobe, Japan). In brief, lysate of frozen material was applied to a well of a dot-blot device. Expression of CDKs was detected quantitatively by sequential reactions with primary anti-CDK antibodies, biotinylated anti-rabbit antibodies, and fluorescein-labelled streptavidin. To measure kinases activity, each CDK molecule was immunoprecipitated from the tissue lysate. The thiophosphate of ATP-γS was transferred to the protein substrate during the on-bead kinases reaction. The introduced thiophosphate was labelled further with 5-iodoacetamidofluorescein and blotted onto a polyvinylidene fluoride membrane. The kinases activity was determined by measuring the fluorescence intensity of the blot.
CDK SA was calculated as kinases activity (mAU per μl lysate) divided by its corresponding mass concentration (EU per μl lysate). Both of AU (CDK activity unit) and EU (CDK expression unit) were defined as the equivalent expression and activity of 1 ng of recombinant active CDK molecule, respectively.
Risk score
In the previous Japanese study, the distribution of CDK1SA and CDK2SA was moderately related (r=0.501, log (CDK2SA)=0.533log (CDK1SA)+1.225), and the aberration from this relationship correlated with the rate of recurrence (Kim et al, 2008). Besides, recurrences were frequently observed in patients with a tumour with higher CDK1SA. The extent of the aberration was quantified as the ratio of CDK2SA relative to CDK1SA (CDK2SA/CDK1SA). The rate of recurrence monotonically increased with increasing ratio of CDK2SA/CDK1SA or CDK1SA. These plots approximated logistic curves (Equations (1) and (2)) and the RS was defined by combining these relational equations. Setting the cutoff value with the cases of the Japanese study; 40% of the patients in the high RS group, showed a significantly lower recurrence-free survival rate in 5 years after surgery (84.0%; 9 out of 58) compared with 40% of the low RS group (96.5%; 2 out of 58) (P=0.009). Less than 20% of the patients were regarded as an intermediate RS group.
The risk for recurrence was quantified as an RS.



Exclusion from statistical analysis
Severe blood contamination into the tissue lysate impairs the accuracy of the expression analysis. To avoid this problem, the extent of contamination is routinely visually quantified by comparing the redness of the lysate with a standard colour bar, which ranges from dark to faint and is graded 1–10; tissue with grade 1–3 are excluded from analysis. Another sample was excluded due to assay failure. Cellularity of the tissue was judged in the C2P system by the expression of CDKs because the molecule is expressed ubiquitously and continuously during the cell cycle. All samples whose CDK1 or CDK2 expression was below the detection limit of the system (3.2 U μl of lysate for CDK1 and 0.08 U μl of lysate for CDK2) were judged to contain an insufficient number of cells for the system and were excluded from the analysis.
Statistical analysis
Statistical analyses were performed using the statistical package SPSS for Windows 15.0. (SPSS Inc., Chicago, IL, USA). Descriptive data are given as mean (s.d.) or median (range). The relationship between CDK-based risk groups and established prognostic factors were investigated using Pearson's χ2 test. All testing was two-tailed with 0.05 as level of significance (Altman et al, 2000).
Distant Recurrence-Free Period (DRFP) was defined as the time from surgery up to the first date of distant recurrence. Overall Survival was defined from the date of surgery up to the date of death due to any cause. To examine if CDK-based risk correlates with DRFP and OS, univariate Cox analysis was performed. Multivariate analyses were performed using the Cox-proportional hazards model entering CDK with other significant variables (defined as those with P<0.1 on univariate analysis). Distance Recurrence Free Period rates are reported as cumulative incidence functions, after accounting for death as competing risk (Putter et al, 2007).
Role of funding source
This retrospective study was sponsored by an unrestricted educational grant of Sysmex (Kobe, Japan).
Results
Patients
A total of 803 patients with early breast cancer were treated with primary surgery in our centre during the study period. Frozen material was available from 352 out of 803 (44%) patients. Median follow-up of patients alive at last follow up was 15 years (range, 6–21). Clinicopathological and treatment characteristics are shown in Table 1. There were minor differences between patients in which CDK was feasible and in which it was not possible. CDK determination was feasible in those patients who had larger tumours (70 vs 60%), had slightly more node-positive disease (53 vs 47%) and were less treated with breast conserving surgery (24 vs 35%). In general, there were no large differences in survival characteristics.
CDK1- and 2-specific activities in tumour tissue
Determination of CDK-based risk by RS was successful in 52% of patients (184 out of 352). In 48% of cases it was not possible due to extreme blood contamination (n=33), OCT contamination (n=45), assay failure (n=1) or low cellularity (n=79). According to CDK-based risk, 41% (n=76) were classified as low, 13% (n=23) as intermediate, and 46% (n=85) as high CDK-based risk.
CDK-based risk and clinicopathological parameters
Correlation between established clinicopathological variables and CDK-based risk are shown in Table 2. There was a significant association between CDK-based risk and age, nodal status, and grade. High CDK-based risk was increasingly evident in younger patients, node-positive disease and grade-III tumours. There was also an association between histological type and CDK-based risk; however, most tumours were ductal carcinomas. No significant association was found between CDK-based risk and tumour size, hormonal receptors, Ki-67 expression, HER2 expression, and vascular invasion.
CDK-based risk and survival
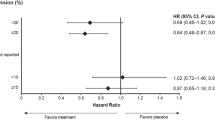
Patients with tumours classified as low or intermediate CDK-based risk showed higher DRFP rates than patients with tumours with high CDK-based risk (intermediate and high-risk group vs low-risk group; hazard ratio (HR) 1.50; 95% confidence intervals (CI) 0.74–3.05 and HR=2.04; 95% CI 1.26–3.28, respectively, overall P-value=0.014) (Figure 1). If we compare the low vs high CDK-based risk group concerning DRFP at 5, 10 and 15 years, 95% CIs are 0.04–0.34, 0.07–0.38 and 0.06–0.38, respectively. Patients with a low CDK-based risk have a better OS than patients with a high CDK-based risk, although this difference is not statistically significant (intermediate and high-risk group vs low-risk group; HR=0.94; 95% CI 0.49–1.79 and HR=1.37; 95% CI 0.92–2.05, respectively, overall P-value=0.216).
All variables considered important for DRFP were analysed in Cox analysis (Table 3). In multivariate Cox-regression analysis, positive nodal status, mastectomy with radiotherapy, no systemic treatment, and high CDK-based risk were predictive for a decreased DRFP.
Prognostic value of CDK-based risk
To examine the prognostic value of CDK-based risk, we excluded all patients who received systemic treatment. This patient population could be categorised by CDK-based risk as: low 44% (n=43 out of 97), intermediate 16% (n=15 out of 97), and high 40% (n=39 out of 97). Patients with tumours classified as low or intermediate CDK-based risk showed higher DRFP rates than patients with tumours with high CDK-based risk (intermediate and high-risk group vs low-risk group; HR 1.40; 95% CI 0.49–3.99 and HR=2.31; 95% CI 1.13–4.73, respectively, overall P-value=0.068) (Figure 2). If we compare the low vs high CDK-based risk group, differences in DRFP at 5, 10, and 15 years are 19, 28 and 26%, respectively. Accompanying 95% CIs for these differences are 0.01–0.39, 0.06–0.48, and 0.01–0.47, respectively (Figure 2). There was no statistical difference between these groups concerning OS (intermediate and high-risk group vs low-risk group; HR=1.06; 95% CI 0.47–2.37 and HR=1.44; 95% CI 0.81–2.54, respectively, overall P-value=0.432).
Univariate and multivariate Cox-regression analysis showed that CDK-based risk groups remained statistically significant (Table 4). Factors associated with a shorter DRFP were positive lymph nodes, mastectomy with radiotherapy, and high CDK-based risk.
Discussion
In our European patient population with early breast cancer, the CDK-based risk was validated. Multivariate analysis showed that CDK-based risk was an independent significant prognostic factor for DRFP in all patients and in patients treated with local therapy only. CDK-based risk is a tangible prognostic marker for DRFP.
Currently, risk-assessments with various prognostic and predictive markers are used for indication of systemic treatment, like tumour grade and nodal status for general systemic treatment choice, hormonal receptors for hormonal treatment, and HER2 expression for immunotherapy. However, these assessments are insufficient for optimal therapeutic decision, especially when applied to node-negative early breast cancer patients. Only few of these patients are considered at such a low-risk of relapse that systemic therapy can be avoided. At the same time, not all patients at high-risk experience a recurrence. Therefore, there is demand for more accurate prognostic markers for a more tailored definition of an individual patient's risk of disease recurrence and to identify indications for the best therapy.
In 2007, the American Society of Clinical Oncology Committee recommended the following markers in clinical practice in patients with early breast cancer: ER, PgR, HER2, urokinases plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1), and certain genes detected with multiparameter gene expression assays (Harris et al, 2007). ER, PgR, and HER2 are widely used and should be determined in every patient with early breast cancer. uPA and PAI-1 are key factors in efficient focal proteolysis, adhesion, and migration of tumour cells (Andreasen et al, 1997; Schmitt et al, 1997; Bouchet et al, 1999; Duffy, 2002; Harbeck et al, 2002). Currently, the prognostic value of uPA and PAI-1 are being examined in the prospective Node-Negative Breast Cancer III (NNBC 3)-Europe Trial (Annecke et al, 2008). As another prognostic tool, the value of microarray-based prognostics and feasibility of its clinical application into clinical practice is in the process of evaluation by two major trials. The first prospective trial is the European Microarray in Node-Negative Disease May Avoid Chemotherapy Trial evaluating MammaPrint (Agendia, Amsterdam, the Netherlands), a 70-gene expression profile, in node-negative early breast cancer patients (van de Vijver et al, 2002; Cardoso et al, 2008). Its American counterpart, the Trial Assigning Individualized Options for Treatment, is aimed at validating Oncotype DX (Genomic Health, Redwood City, CA, USA), a 21-gene assay, likewise in node-negative patients (Palli et al, 1999; Sparano and Paik, 2008). The Oncotype DX profile can be determined using paraffin-embedded breast tissue; the MammaPrint profile makes use of fresh frozen material. Both profiles must be analysed centrally; no ready-to-use kit is available to determine the profile in local hospitals.
In the ideal clinical trial setting, the above-mentioned prognostic factors, including CDK-based risk, should be determined in the same tumour sample to determine the best marker combination for optimal treatment decisions. Unfortunately it is not likely that such a large, long-lasting and costly trial will be actualised.
From our results, it was shown that validation of CDK-based risk was feasible for European patients even though the RS was determined in Japanese patients. Despite the difference between the cohorts, it may be concluded that CDK-based risk is a new prognostic factor. However, before this CDK-based risk can be used in daily clinical practice, several aspects have to be considered. First issue is the high proportion of exclusion cases in this study; in almost 50% of tumours it was not feasible to determine CDK-based risk. This was related to weaknesses of the assay system, because the accuracy of the expression analysis is influenced by blood and OCT contamination. However, we believe these issues are not significant problems anymore. A washing step before tissue lysis markedly improved the efficacy of CDK expression analysis for cases with ⩾20% OCT contamination (data not shown). The crucial issue of the exclusion is low cellularity measured by CDK expression. In our study, 22% (79 out of 352) were excluded because of low cellularity. The Japanese retrospective study, which used snap-frozen tissues, found a lower rate of low cellularity (11%, unpublished data), and this value was judged as a clinically practical value. One challenge is to carefully determine the sufficient amount of a cryosection to apply CDK-based risk on OCT-embedded samples. Secondly, to enable general use for broad application, a feasible ready-to-use kit should be devised including the cell cycle profile system.
In the association analysis of CDK-based risk to clinicopathologic factors, we found significant associations between the CDK-based risk and age, nodal status, and grade. Unexpectedly, a statistically significant association was not observed between CDK-based risk and Ki67 expression, known as a proliferation marker of tumour tissues. This discrepancy may be because of the technical issue of Ki67 immunohistochemistry procedure; the lack of an international standardisation method for antigen retrieval, staining procedures and scoring methods. To further address the significance of CDK-based analysis, cell biological interest for rate of cell proliferation, cell cycle distribution (G1/S/G2M-phase), check point regulation, and CDK-mediated cell death should be examined both in vitro and in vivo.
In conclusion, our results showed that CDK-based risk is prognostic for DRFP in patients with early breast cancer, also after correction with other prognostic factors. Therefore, further studies are justified to develop this as a marker for more tailored treatment of early breast cancer patients.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Altman DG, Machin D, Bryant TN, Gardner MJ (2000) Statistics with Confidence. British Medical Journal Books: Bristol
Andreasen PA, Kjoller L, Christensen L, Duffy MJ (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72: 1–22
Annecke K, Schmitt M, Euler U, Zerm M, Paepke D, Paepke S, von Minckwitz G, Thomssen C, Harbeck N (2008) uPA and PAI-1 in breast cancer: review of their clinical utility and current validation in the prospective NNBC-3 trial. Adv Clin Chem 45: 31–45
Bouchet C, Hacene K, Martin PM, Becette V, Tubiana-Hulin M, Lasry S, Oglobine J, Spyratos F (1999) Dissemination risk index based on plasminogen activator system components in primary breast cancer. J Clin Oncol 17: 3048–3057
Cardoso F, Van't Veer L, Rutgers E, Loi S, Mook S, Piccart-Gebhart MJ (2008) Clinical application of the 70-gene profile: the MINDACT trial. J Clin Oncol 26: 729–735
Duffy MJ (2002) Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies. Clin Chem 48: 1194–1197
Early Breast Cancer Trialists' Collaborative Group (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717
Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG (1997) Overexpression of cyclin D1 in human pancreatic carcinoma is associated with poor prognosis. Cancer Res 57: 1634–1637
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 54: 1812–1817
Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18: 1133–1144
Gramlich TL, Fritsch CR, Maurer D, Eberle M, Gansler TS (1994) Differential polymerase chain reaction assay of cyclin D1 gene amplification in esophageal carcinoma. Diagn Mol Pathol 3: 255–259
Harbeck N, Kates RE, Schmitt M (2002) Clinical relevance of invasion factors urokinase-type plasminogen activator and plasminogen activator inhibitor type 1 for individualized therapy decisions in primary breast cancer is greatest when used in combination. J Clin Oncol 20: 1000–1007
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast Jr RC (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25: 5287–5312
Ishihara H, Yoshida T, Kawasaki Y, Kobayashi H, Yamasaki M, Nakayama S, Miki E, Shohmi K, Matsushima T, Tada S, Torikoshi Y, Morita M, Tamura S, Hino Y, Kamiyama J, Sowa Y, Tsuchihashi Y, Yamagishi H, Sakai T (2005) A new cancer diagnostic system based on a CDK profiling technology. Biochim Biophys Acta 1741: 226–233
Kim SJ, Nakayama S, Miyoshi Y, Taguchi T, Tamaki Y, Matsushima T, Torikoshi Y, Tanaka S, Yoshida T, Ishihara H, Noguchi S (2008) Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol 19: 68–72
Nakashima S, Natsugoe S, Matsumoto M, Kijima F, Takebayashi Y, Okumura H, Shimada M, Nakano S, Kusano C, Baba M, Takao S, Aikou T (2000) Expression of p53 and p21 is useful for the prediction of preoperative chemotherapeutic effects in esophageal carcinoma. Anticancer Res 20: 1933–1937
Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, Pacini P (1999) Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. National Research Council Project on Breast Cancer Follow-up. JAMA 281: 1586
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26: 2389–2430
Schmitt M, Harbeck N, Thomssen C, Wilhelm O, Magdolen V, Reuning U, Ulm K, Hofler H, Janicke F, Graeff H (1997) Clinical impact of the plasminogen activation system in tumor invasion and metastasis: prognostic relevance and target for therapy. Thromb Haemost 78: 285–296
Sjostrom J, Blomqvist C, Heikkila P, Boguslawski KV, Raisanen-Sokolowski A, Bengtsson NO, Mjaaland I, Malmstrom P, Ostenstadt B, Bergh J, Wist E, Valvere V, Saksela E (2000) Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy response in advanced breast cancer. Clin Cancer Res 6: 3103–3110
Sparano JA, Paik S (2008) Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol 26: 721–728
Sutter T, Doi S, Carnevale KA, Arber N, Weinstein IB (1997) Expression of cyclins D1 and E in human colon adenocarcinomas. J Med 28: 285–309
Takano Y, Kato Y, van Diest PJ, Masuda M, Mitomi H, Okayasu I (2000) Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am J Pathol 156: 585–594
van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AAM, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers EJTh, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347: 1999–2009
van Diest PJ, Michalides RJ, Jannink L, van d V, Peterse HL, de Jong JS, Meijer CJ, Baak JP (1997) Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol 150: 705–711
Acknowledgements
We thank Klaas van der Ham for his help with the database and thank Lambert van den Broek for his help with the frozen tumour material. We thank Jörn Kirchhuebel for reading the paper. We thank Sysmex for the unrestricted educational grant. Presented in part as poster presentation at the 30th San Antonio Breast Cancer Symposium, 2007, San Antonio, Texas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
van Nes, J., Smit, V., Putter, H. et al. Validation study of the prognostic value of cyclin-dependent kinase (CDK)-based risk in Caucasian breast cancer patients. Br J Cancer 100, 494–500 (2009). https://doi.org/10.1038/sj.bjc.6604870
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604870
Keywords
This article is cited by
-
Cyclin-dependent kinase-specific activity predicts the prognosis of stage I and stage II non-small cell lung cancer
BMC Cancer (2014)
-
High nuclear/cytoplasmic ratio of Cdk1 expression predicts poor prognosis in colorectal cancer patients
BMC Cancer (2014)
-
The prognostic value of apoptotic and proliferative markers in breast cancer
Breast Cancer Research and Treatment (2013)
-
Specific activity of cyclin-dependent kinase I is a new potential predictor of tumour recurrence in stage II colon cancer
British Journal of Cancer (2012)