Abstract
The xc− cystine transporter enhances biosynthesis of glutathione, a tripeptide thiol important in drug resistance and cellular defense against oxidative stress, by enabling cellular uptake of cystine, a rate-limiting precursor. Because it is known to regulate glutathione levels and growth of various cancer cell types, and is expressed in the pancreas, we postulate that it is involved in growth and drug resistance of pancreatic cancer. To examine this, we characterised expression of the xc− transporter in pancreatic cancer cell lines, MIA PaCa-2, PANC-1 and BxPC-3, as subjected to cystine-depletion and oxidative stress. The results indicate that these cell lines depend on xc−-mediated cystine uptake for growth, as well as survival in oxidative stress conditions, and can modulate xc− expression to accommodate growth needs. Immunohistochemical analysis showed that the transporter was differentially expressed in normal pancreatic tissues and overexpressed in pancreatic cancer tissues from two patients. Furthermore, gemcitabine resistance of cells was associated with elevated xc− expression and specific xc− inhibition by monosodium glutamate led to growth arrest. The results suggest that the xc− transporter by enhancing glutathione biosynthesis plays a major role in pancreatic cancer growth, therapy resistance and represents a potential therapeutic target for the disease.
Similar content being viewed by others
Main
Pancreatic cancer is the second most common gastrointestinal malignancy and the fourth leading cause of cancer-related deaths in the United States (Freelove and Walling, 2006). It is highly resistant to conventional chemotherapeutic regimens (Zalatnai and Molnar, 2007) and gemcitabine (GEM), currently the standard chemotherapeutic agent for treatment of pancreatic cancer, is only marginally effective (Wolff, 2007). Clearly, there is a critical need for establishing new targets and approaches for therapy of pancreatic cancer.
Glutathione (GSH) is a tripeptide thiol consisting of glutamate, cysteine and glycine, which plays a critical role in cellular defenses against oxidative stress and toxic compounds (Griffith, 1999). In cancer cells the GSH levels maintain DNA synthesis, growth and multidrug/radiation resistance, and sustenance of GSH levels through GSH biosynthesis is vital for growth and survival of tumours. As such, GSH is considered an important target in cancer therapy and various therapeutic approaches based on GSH depletion of cancer cells have been suggested (Schnelldorfer et al, 2000; Estrela et al, 2006; Doxsee et al, 2007). Glutathione biosynthesis is critically dependent on availability of intracellular cysteine, a major rate-limiting factor (Griffith, 1999). Although tissues such as the liver can synthesise cysteine from L-methionine through the transsulphuration pathway (Rosado et al, 2007), certain experimental cancers (e.g., lymphomas, gliomas) are incapable of synthesising adequate amounts of the amino acid for GSH synthesis and hence depend for growth and viability on uptake of extracellular cysteine or cystine, the oxidised form. In such cases, reduced cellular uptake of the amino acid can lead to depletion of intracellular GSH levels and subsequent growth arrest (Gout et al, 1997; Iwata et al, 1997; Chung et al, 2005).
The xc− cystine/glutamate antiporter is a plasma membrane transporter mediating cellular uptake of cystine in exchange for intracellular glutamate with a stoichiometry of 1 : 1 (Lo et al, 2008). It is a member of a family of heterodimeric amino-acid transporters, plasma membrane proteins composed of a heavy subunit and a light subunit coupled through a disulphide bridge. The human xc− transporter consists of the heavy subunit 4F2hc, found in a variety of amino-acid transporters, coupled to the light subunit, xCT, which confers specificity for cystine (Verrey et al, 2004). It has been demonstrated, both in vitro and in vivo that inhibition of the xc− transporter in cancer cells depending on extracellular cystine/cysteine for growth and viability, can lead to reduced uptake of this amino acid with subsequent depletion of intracellular GSH levels and growth arrest (Gout et al, 2001; Chung et al, 2005; Doxsee et al, 2007). For such malignancies the xc− transporter therefore represents a potential therapeutic target (Lo et al, 2008).
Human xCT is expressed in the pancreas, as one of a limited number of tissues (Bassi et al, 2001). Although xc− activity has been investigated in normal pancreatic cell lines (Sato et al, 1998), the function of the transporter in pancreatic cancer cells has so far not attracted much attention. In the present study, we have investigated a potential role for the xc− transporter in growth, viability and GSH-based drug resistance of pancreatic cancer, using human pancreatic cancer cell lines, MIA PaCa-2, PANC-1, and BxPC-3, in particular as modulated by oxidative stress factors, changes in extracellular cystine levels and specific xc− inhibition. Importantly, pancreatic cancer tissues from patients showed overexpression of the transporter. Taken together, the results suggest that the xc− cystine transporter plays a significant role in the growth, viability and therapy resistance of pancreatic cancers and may be useful as a potential target for therapy of the disease.
Materials and methods
Materials
Chemicals, dyes, solvents, solutions and culture medium were obtained from Sigma-Aldrich Canada Ltd, Oakville, ON, Canada, unless otherwise indicated.
Maintenance of pancreatic cancer cell cultures
The human pancreatic cancer cell lines, MIA PaCa-2, PANC-1 and BxPC-3, were originally obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained as monolayer cultures in T75 tissue culture flasks (Nunc™, Roskilde, Denmark) in MEM containing 0.1 mM cystine (StemCell Technologies, Vancouver, BC, Canada), supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA) and 3.6 g l−1 glucose at 37°C in a humidified chamber in an atmosphere of 95% air and 5% CO2.
Assessment of growth requirements for exogenous cystine
Cells were plated in 96-well plates (Nunc™) at 1000 cells per well (in triplicate) in their maintenance medium overnight. Cells were then washed and overlaid with cystine- and methionine-deficient RPMI-1640 medium supplemented with 10% FBS and 2 mM glutamine, in the presence or absence of cystine (0.1 mM), methionine (0.1 mM), and/or cystathionine (0.15 mM) in various combinations. Following 72 h of incubation, a neutral red uptake assay was performed to determine cell survival. It is noteworthy that normal cystine concentrations in human blood plasma range between 0.1–0.2 mM (Chawla et al, 1984; Gmunder et al, 1991). Therefore, we defined the normal cystine concentration to be 0.1 mM cystine. Accordingly, cystine concentrations lower than 0.1 mM or higher than 0.2 mM were considered as low or high cystine concentrations, respectively.
Cell survival/proliferation assay
Cell proliferation and viability were determined by neutral red uptake (Babich and Borenfreund, 1991). Cells were plated in 96-well plates at 1000 cells per well. The next day, cells were treated as dictated by the particular experiments. Wells were then aspirated and incubated with 100 μl 0.0025% neutral red dye in cell culture medium. Empty wells were also incubated with neutral red dye to allow for background absorbance correction. After 4 h of incubation, wells were aspirated and 100 μl 1% acetic acid in 50% ethanol was added per well to solubilise intracellular neutral red dye. Absorbance was determined at 550 nm using a 96-well VERSA max microplate reader (Molecular Devices, Sunnyvale, CA, USA).
RNA isolation and q-RT–PCR
Isolation of total RNA from cultured cells in vitro or tumour cells in vivo was performed using an RNeasy Micro kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer's recommendations. RNA (5 μg) was used to synthesise first-strand cDNA using the Superscript II RT–PCR kit (Invitrogen), according to the manufacturer's recommendations. q-RT–PCRs were performed in 384-well plates using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in a volume of 15 μl (containing 50 ng of cDNA). Fluorescence emission was detected for each PCR cycle on a ABI Prism® 7900 Sequence Detection System (Applied Biosystems) and threshold cycle (Ct) values were determined based on a 40-cycle reaction. Thermal cycling conditions were 50°C for 2 min and 95°C for 5 min, followed by 40 cycles of 15 s at 95°C, 30 s at 58°C, and 30 s at 72°C. Average Ct values from duplicate PCRs were normalised to average Ct values for the housekeeping gene β-2-microglobulin (β2M) from the same cDNA preparations. The relative mRNA expression of a gene was calculated as follows: Average Ct (β2M)−Average Ct (gene)=d; mRNA expression relative to β2M=2^(d). Each q-RT–PCR was carried out in duplicate.
Primer sequences are as follows: mouse 4F2hc: forward 5′-GAGGACAGGCTTTTGATTGCAG-3′, reverse 5′-AGGTAGGAGCTGGTCAACAGCA-3′; mouse xCT: forward 5′-GAGGACAGGCTTTTGATTGCAG-3′, reverse 5′-AGGTAGGAGCTGGTCAACAGCA-3′; mouse β2M: forward 5′-CACCCCCACTGAGAGACTGATACA-3′, reverse 5′-TGATGCTTGATCACATGTCTCG-3′; human 4F2hc: forward 5′-AGTGCCAACATGACTGTGAAG-3′, reverse 5′-CCTTACTCCGCTGGTCACTCAG-3′; human xCT: forward 5′-TGCTGGCTGGTTTTACCTCAAC-3′, reverse 5′-CCAATGGTGACAATGGCCAT-3′; human β2M: forward 5′-ACCATGTGACTTTGTCACAGCC-3′, reverse 5′-AATCCAAATGCGGCATCTTC-3′.
Glutathione assay
Total GSH levels were measured using the ApoGSH™ GSH Colorimetric Detection Kit (BioVision, Mountain View, CA, USA) according to the manufacturer's recommendations.
Western blotting
Total cellular protein was extracted on ice by sonication and supplemented with a protease inhibitor solution (one tablet of Complete Protease Inhibitor Cocktail dissolved in 25 ml of 1 × PBS) (Roche, Basel, Switzerland).
Protein samples (10 μg) were electrophoresed under reducing conditions on NuPAGE 4–12% bis-Tris gels and transferred to 0.45-μm nitrocellulose membranes (Invitrogen). Membranes were blocked overnight at 4°C with 10% skim milk in 1 × PBS, followed by incubation with the following primary antibodies: mouse anti-human 4F2hc (Chemicon, Temecula, CA, USA; 1 : 250 dilution) and mouse anti-human α-tubulin (Sigma, 1 : 10 000 dilution). After incubation for 1 h, membranes were washed and incubated with the secondary antibodies: horse radish peroxidase-linked anti-mouse (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; 1 : 10 000 dilution) or horse radish peroxidase-linked anti-rabbit IgG (Jackson ImmunoResearch Laboratories; 1 : 2000 dilution). After incubation for 1 h, Amersham (GE Healthcare, Piscataway, NJ, USA) ECL Plus™ western blotting detection reagents were used for visualisation.
Immunofluorescence microscopy
Pancreatic cancer cells used for immunocytochemistry were cultured on glass chamber slides. Cells were washed two times in PBS and fixed in 4% paraformaldehyde for 10 min. Following three washes in PBS and 30 min incubation in blocking solution (PBS plus 5% goat serum plus 0.1% Triton X-100), cells were incubated with rabbit anti-mouse xCT antibody (Transgenic Inc., Kumamoto, Japan; 1 : 50 dilution) or mouse anti-human 4F2hc antibody (Chemicon; 1 : 200 dilution), diluted in blocking solution for 45 min at 4°C. Cells were then rinsed three times with blocking solution and incubated with either goat anti-mouse or goat anti-rabbit AlexaFluor®594 (Invitrogen; 1 : 100 dilution) for 30 min in the dark. Finally, cells were washed, stained with 4′6-diamidino-2-phenylindole (DAPI) and mounted with Vectashield® (Vector Laboratories, Burlingame, CA, USA). Immunofluorescence was detected with an Axiovert 40 imaging microscope (Carl Zeiss Canada, Toronto, ON, Canada), and images were captured with a AxioCam HRC digital camera (Carl Zeiss). Merged images were generated using Adobe PhotoShop (Adobe Systems, Mountain View, CA, USA).
Primary human pancreatic tumours and corresponding normal human pancreatic tissues (gift of Dr Sylvia Ng, BC Cancer Agency, Vancouver, BC, Canada.) were embedded and frozen in Tissue-Tek® optimal cutting temperature compound (Somagen Diagnostics, Edmonton, Alberta, Canada), sectioned at 5-μm thickness using a CM1850 UV cryostat (Leica, Wetzlar, Germany), and mounted on Fisherbrand Plus microscope slides. Subsequently, these samples were subjected to the same immunohistochemical staining procedures as described for cultured cells. The patient samples were obtained in agreement with the Ethical Committee of the University of British Columbia and after obtaining informed consent from the patients.
Transient transfection
Plasmid constructs of mouse xCT and 4F2hc cDNAs cloned into the pcDNA3.1+ vector were transformed into the E.coli strain DH5α, and plasmid DNA harvested using a Qiagen Maxiprep kit according to the manufacturer's recommendations.
For xc− transporter overexpression studies, pancreatic cancer cells were plated at a density of 0.5 × 103 cells per well in 96-well plates. After 24 h, wells were aspirated and 125 μl of fresh media were added. Cells were transiently transfected with 0.1 μg DNA (empty vector pcDNA3.1 control, pcDNA3.1-xCT, or pcDNA3.1-4F2hc) diluted in 12.5 μl 150 mM NaCl and 0.75 μl ExGen 500 in vitro transfection reagent (Fermentas, Burlington, ON, Canada) for 24 h.
Glutamate uptake assay
Glutamate uptake by cultured cells was used to measure xc− transporter activity as described previously (Shih and Murphy, 2001). Briefly, cells were plated in 24-well plates at 5 × 105 cells per well and incubated overnight. Cells were washed with and pre-incubated in 1 ml per well Na+-free buffer A consisting of 140 mM N-methyl-D-glucamine, 5.4 mM KCl, 0.4 mM KH2PO4, 10 mM HEPES, 5 mM D-glucose, 1.8 mM CaCl2, 0.8 mM MgSO4 (pH 7.4) for 20 min at 37°C. The medium was then replaced with 300 μl Na+-free buffer A containing 33 nM L-[3H]-glutamate (49 Ci mmol−1) (Amersham Pharmacia/GE Healthcare, Pittsburg, PA, USA) in the presence or absence of 1 μ M unlabeled amino-acid competitors (L-glutamate, L-cystine) or non-competitors (L-leucine) for 20 min at 37°C. Uptake was terminated by washing three times with ice-cold Na+-free buffer A, after which cells were solubilised with 200 μl 0.5% Triton X-100 in 0.1 M potassium phosphate buffer (pH 7.0). To determine intracellular L-[3H]-glutamate uptake, 100 μl of cell lysate was mixed with 5 ml of scintillation cocktail (Fisher, Pittsburg, PA, USA), and radioactivity was measured using an LKB Wallac 1214 Rackbeta (American Instrument Exchange Inc., Haverhill, MA, USA) liquid scintillation counter. A 10 μl aliquot of cell lysate was used in a BCA protein assay kit (Pierce Chemical Co, Rockford, IL, USA) to determine protein concentration.
Statistical analysis
Student's t-test was used unless otherwise stated to determine statistical significance. Results with a P⩽0.05 were considered significant.
Results
Growth requirement for extracellular cystine
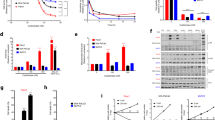
It is known that cysteine, the reduced form of cystine, is a non-essential amino acid in the human diet as tissues in the body such as the liver can generate cysteine through the transsulphuration pathway (Stabler et al, 1993; Brosnan and Brosnan, 2006). This pathway involves the metabolism of methionine, a nutritionally essential amino acid, to a cystathionine intermediate and its subsequent cleavage by γ-cystathionase to α-ketobutyrate and cysteine (Stabler et al, 1993; Brosnan and Brosnan, 2006). Certain cancer cells cannot generate their own cysteine/cystine and hence depend on extracellular sources to sustain growth and survival (Iglehart et al, 1977; Gout et al, 2001). To determine if pancreatic cancer cells require exogenous cystine for growth and survival, we cultured MIA PaCa-2, PANC-1, and BxPC-3 in the presence and absence of cystine, methionine, and/or cystathionine in all possible combinations. Survival and robust growth of all three cancer cell lines was observed only in cultures containing both methionine and cystine (Figure 1), demonstrating that the absence of either amino acid inhibited survival and proliferation in vitro. Cystathionine, which can substitute for cystine in some cell systems (Uren and Lazarus, 1979), failed to promote cell survival/growth when added to cystine-deficient, methionine-containing cultures (Figure 1). These results indicate that pancreatic cancer cell lines are dependent on uptake of cystine from their microenvironment for growth and survival, and suggest that the enzymes involved in the transsulphuration pathway may not be present or activated in these cells.
Pancreatic cancer cells depend on extracellular cystine for growth. Neutral red uptake assay for cell proliferation in MIA PaCa-2, PANC-1, and BxPC-3 cells incubated in medium in the presence or absence of methionine (0.1 mM), cystine (0.1 mM), and/or cystathionine (0.15 mM), in the combinations indicated for 72 h. Data represent the mean±s.e.m. from three independent experiments. *P<0.01.
A negative correlation exists between extracellular cystine deprivation and expression of the xc− transporter
The xc− transporter is a major transporter of extracellular cystine (Bannai, 1984b). To determine whether extracellular cystine concentrations affect the expression of the xc− transporter, pancreatic cancer cells were incubated in a medium containing low (0.01 mM), normal (0.1 mM) or high (1.0 mM) levels of cystine for up to 72 h. Cells in media containing low cystine exhibited signs of death after 72 h (data not shown). The xc− transporter is structurally composed of an xCT light subunit, which confers substrate specificity and a 4F2hc heavy subunit, which is a common subunit of many amino-acid transporters (Sato et al, 1999; Bassi et al, 2001). The mRNA expression levels of the xCT and 4F2hc subunits at varying cystine concentrations were determined by quantitative real-time RT–PCR (q-RT–PCR). In response to low cystine concentrations, xCT mRNA was elevated in two of the three cell lines (MIA PaCa-2 and PANC-1), whereas 4F2hc mRNA was elevated in all three cell lines (MIA PaCa-2, PANC-1 and BxPC-3) (Figure 2A). Expression of 4F2hc protein in all three cell lines was determined by western blot analysis and yielded a similar increased expression level in response to low cystine concentration (Figure 2B). Unfortunately, no satisfactory xCT antibody for western blotting was available at the time these experiments were conducted, thus precluding the examination of xCT protein expression in our studies. These findings demonstrate that inverse correlations exist between extracellular cystine concentration and both xCT and 4F2hc expression in some pancreatic cancer cell lines, suggesting that these cells can modulate the expression of xc− transporter to accommodate their growth needs.
A negative correlation exists between extracellular cystine deprivation and expression of the xc− transporter. (A) Q-RT–PCR for expression of xCT or 4F2hc mRNA in MIA PaCa-2, PANC-1, and BxPC-3 cell lines incubated with various cystine concentrations (0.01, 0.1 or 1 mM) for 72 h. Data represent the mean±s.e.m. from three independent experiments. *P⩽0.05. (B) Western blot for expression of 4F2hc or tubulin in MIA PaCa-2, PANC-1, and BxPC-3 cell lines incubated with various cystine concentrations (0.01, 0.1 or 1 mM) for 72 h. Results are representative of two independent experiments.
Oxidative stress increases xc− transporter expression and GSH levels
The oxidative stressor, diethylmaleate (DEM), is commonly used to regulate intracellular GSH levels (Bannai, 1984a; Kim et al, 2001; Hosoya et al, 2002) and is often used to induce the expression of stress response-related genes. All three pancreatic cancer cell lines were treated with 1 mM DEM for 24 h, and an increase in total intracellular GSH levels in response to DEM treatment was confirmed (Figure 3A). In a blood–brain barrier cell line, treatment with DEM increased GSH levels with a corresponding increase in xCT mRNA expression (Hosoya et al, 2002). To determine whether the DEM-induced increase in GSH levels in pancreatic cancer cells corresponded with an increase in xc− transporter expression, mRNA expression levels of xCT and 4F2hc were determined by q-RT–PCR. xCT mRNA expression was significantly upregulated in all three cell lines in response to DEM (Figure 3B). In contrast, 4F2hc mRNA remained at control levels (Figure 3B), consistent with previous studies that reported no effect of DEM on 4F2hc mRNA expression in a rat retinal capillary endothelial cell line (Tomi et al, 2002) and a human retinal pigment epithelial cell line (Bridges et al, 2001). Corresponding 4F2hc protein levels also remained unchanged in response to DEM treatment (Figure 3C). To assess xCT protein expression, immunofluorescent staining with an anti-xCT antibody was performed on pancreatic cancer cell lines. Increased xCT protein levels were observed in all three pancreatic cancer cell lines in response to DEM treatment, with the BxPC-3 cell line exhibiting a clear localisation of the xCT protein to the plasma membrane upon treatment with DEM (Figure 3D). These findings suggest that pancreatic cancer cells, in response to oxidative stress, upregulate expression of the xc− transporter by inducing xCT (but not 4F2hc) subunit expression, resulting in a corresponding increase in GSH synthesis. This increase in GSH synthesis may in turn enable pancreatic cancer cells to survive in the presence of elevated levels of reactive oxygen species.
Oxidative stress increases xc− transporter expression and GSH levels. (A) Intracellular GSH levels in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 mM DEM for 24 h. Data represent the mean±s.e.m. from at least two independent experiments. *P<0.05 using the Wilcoxon rank-sum test. (B) Q-RT–PCR for xCT and 4F2hc mRNA expression in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 mM DEM for 24 h. Data represent the mean±s.e.m. from at least two independent experiments. *P<0.05 (C) Western blot for expression of 4F2hc and tubulin in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 mM DEM for 24 h. Results are representative of two independent experiments. (D) Immunofluorescence for xCT (red) and DAPI (blue) in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 mM DEM for 24 h. Results are representative of two independent experiments.
Expression of the xc− transporter in primary human pancreatic cancer specimens
Having demonstrated that pancreatic cancer cell lines express the xc− transporter, its expression was assessed in primary human pancreatic cancer tissues. Owing to difficulties in obtaining fresh human pancreatic cancer tissues, only two patient samples were used in this study. Both pancreatic cancer patient specimens with corresponding normal pancreatic tissue from the same patient were stained with anti-xCT antibody by immunofluorescence. Use of a rabbit IgG antibody as a negative control indicated that the xCT staining was not due to non-specific binding of immunoglobulins. In the normal pancreatic tissues, xCT protein expression was primarily localised to ductal cells, not acinar cells (Figure 4). Importantly, the pancreatic ductal adenocarcinomas exhibited overexpression of xCT protein. The distinct histological features of the two pancreatic cancer specimens, including atypical epithelial architecture, papillary folding, and intraluminal cell shedding, indicated that these specimens were likely invasive pancreatic ductal adenocarcinomas. The upregulated expression of xCT protein in the pancreatic cancer tissues suggest that elevated xc− transporter expression plays a role in the pathogenesis of pancreatic cancer.
A positive correlation exists between the expression level of xCT and resistance towards GEM
Thus far we have obtained evidence that elevated xc− transporter expression can enhance pancreatic cancer cell growth by promoting the uptake of extracellular cystine, which in turn functions to maintain high levels of intracellular GSH to promote cancer cell survival in the presence of oxidative stress. Among the three pancreatic cancer cell lines used in this study, PANC-1 cells expressed the highest relative level of xCT mRNA compared with that of BxPC-3 and MIA PaCa-2 (Figure 5A), suggesting that PANC-1 cells may exhibit the greatest resistance towards oxidative stress-induced cell death. Gemcitabine, the most common chemotherapeutic agent for the treatment of pancreatic cancer (Maehara et al, 2004), induces cell death through a mechanism that can involve oxidative stress (Maehara et al, 2004; Donadelli et al, 2007). To determine whether a relationship exists between the expression level of the xc− transporter and resistance towards GEM, we treated pancreatic cancer cells with GEM and determined the half maximal inhibitory concentration (IC50) of GEM using neutral red assays. The IC50 of GEM was highest in PANC-1 cells (>50 μ M) compared with MIA PaCa-2 (∼0.03 μ M) and BxPC-3 (0.01 μ M) cells (Figure 5B). The higher resistance of the PANC-1 cells to GEM may correspond with the higher number of cystine transporters per cell as suggested by relative xCT expression levels. Hence a negative correlation exists between the expression level of xCT and sensitivity to GEM; PANC-1 cells, which have highest relative expression of xCT, are the least sensitive to GEM, whereas MIA PaCa-2 cells, which have the lowest relative expression of xCT, are the most sensitive to GEM.
A positive correlation exists between the expression level of xCT and resistance towards GEM. (A) Q-RT–PCR for xCT expression in MIA PaCa-2, PANC-1, and BxPC-3 cell lines. Data represent the mean±s.e.m. from three independent experiments. *P<0.05. (B) Neutral red uptake assay for cell proliferation in Mia PaCa-2, PANC-1, and BxPC-3 cultures treated with increasing concentrations of GEM for 72 h. Data represent the mean±s.e.m. from three independent experiments. *P<0.01.
Overexpression of transfected xCT increases cystine uptake and increases GEM resistance
To determine if overexpression of the xc− transporter can increase resistance to GEM, xCT and 4F2hc cDNAs were transfected into the pancreatic cell lines MIA PaCa-2 and PANC-1. Increased xCT and 4F2hc mRNA expression was confirmed by semi-quantitative RT–PCR and q-RT–PCR in transfected MIA PaCa-2 (Figure 6A–C) and PANC-1 (data not shown) cell lines. Using a radioactive glutamate uptake assay, MIA PaCa-2 cells transfected with either xCT cDNA alone or both xCT and 4F2hc cDNA exhibited increased xc− transport activity (Figure 6D). Transfection of 4F2hc cDNA alone, however, was not sufficient to upregulate xc− transporter activity (Figure 6D). Furthermore, transfection of both xCT and 4F2hc cDNA was required to increase GEM resistance in MIA PaCa-2 cells (Figure 6E). Hence upregulation of the xCT subunit expression is necessary to upregulate xc− transporter activity, with a corresponding increase in GEM resistance. These results suggest that overexpression of the xc− transporter, as seen in primary pancreatic ductal adenocarcinoma specimens, may increase pancreatic cancer cell survival in response to GEM treatment.
Overexpression of transfected xCT increases cystine uptake and increases GEM resistance. (A) RT–PCR for xCT and 4F2hc expression in MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, or pcDNA3.1-4F2hc plasmid. (B, C) Q-RT–PCR for xCT (B) or 4F2hc (C) expression in MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, or pcDNA3.1-4F2hc plasmid. *P<0.001. (D) [3H]-glutamate uptake assay in MIA PaCa-2 and PANC-1 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, and/or pcDNA3.1-4F2hc plasmid. *P<0.01 compared with respective cell line pcDNA3.1 control. (E) Neutral red uptake assay for survival of untreated or GEM-treated MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, and/or pcDNA3.1-4F2hc plasmid. *P⩽0.05 compared with pcDNA3.1 control; #P⩽0.05 compared with pcDNA3.1 GEM treatment. All data represent the mean±s.e.m. from three independent experiments.
Targeting xc− transporter function with monosodium glutamate
Monosodium glutamate (MSG) is a specific inhibitor of the xc− transporter as the uptake of cystine can be competitively inhibited by glutamate (Bannai, 1986). Consistent with this, pancreatic cancer cells treated with MSG (4.0 mM) exhibited markedly inhibited growth, with growth of MIA PaCa-2 and PANC-1 cultures completely abrogated. Importantly, the growth arrest could be largely prevented by including 2-mercaptoethanol (2-ME; 66 μ M) in the culture medium, a compound commonly used to shuttle cystine into cells through the L-transport system hence circumventing inhibition of the xc− transporter (Ishii et al, 1981) (Figure 7). Taken together, our findings demonstrate that the growth of pancreatic cancer cells can be inhibited by specific inhibition of the xc− transporter.
Discussion
The xc− transporter has been shown in various cancers to supply cells with cystine for GSH synthesis resulting in increased proliferation and GSH-related drug resistance (Doxsee et al, 2007; Narang et al, 2007). The goal of this study was to investigate whether a similar xc−-mediated system was also used by pancreatic cancer cells for growth, survival and drug-related resistance.
In the present study, the human pancreatic cancer cell lines MIA PaCa-2, PANC-1, and BxPC-3 were determined to be critically dependent on extracellular cystine for growth (Figure 1). This finding demonstrates that the biochemical cysteine synthesis pathway known as the transsulphuration pathway does not participate in alleviating cystine depletion in the cell lines of this study. Some cell types, such as neurons and astrocytes, do not rely on the xc− transporter for growth (Chung et al, 2005). As such, these cells may (i) possess a functional transsulphuration pathway, (ii) express other transporters that mediate the transport of cystine or cysteine into the cell, for example, excitatory amino-acid transporters (McBean and Flynn, 2001), or (iii) not express the xc− transporter, but instead rely solely on uptake of extracellular cysteine secreted by certain somatic cells (Gout et al, 2001; Chung et al, 2005; Lo et al, 2008). The potential utility of screening tumour biopsies for the presence of enzymes in the transsulphuration pathway (i.e., for γ-cystathionase) to determine whether a patient may benefit from cystine starvation therapy remains a possibility.
Because our results indicate that pancreatic cancer cell lines are dependent on extracellular cystine/cysteine for growth, these cell lines may exhibit sensitivity towards depletion of the amino acid in their environment. Indeed, at least two of the three cell lines tested showed an inverse correlation between a low extracellular cystine environment (0.01 mM) and expression of the xc− transporter, suggesting that these cells can modulate their expression of the xc− transporter to accommodate their growth needs. Consistent with published reports, cell proliferation is strongly associated with cystine/cysteine availability and intracellular GSH levels (Godwin et al, 1992; Noda et al, 2002).
Besides manipulating cysteine/cystine levels in the extracellular microenvironment to effect a change in xc− transporter expression, the addition of the oxidative stressor DEM was also used. In accordance with other studies (Bannai, 1984a; Kim et al, 2001; Hosoya et al, 2002), DEM treatment induced xCT mRNA expression with a corresponding increase in total GSH, indicating that increased intracellular GSH synthesis enables pancreatic cancer cells to survive in the presence of oxidative stress. In human embryonic kidney (HEK) cells, xCT expression has been reported at the plasma membrane (Shih and Murphy, 2001). Of interest is the BxPC-3 cell line, which upon treatment with DEM, clearly exhibited localisation of the xCT subunit to the plasma membrane (Figure 3D). The 4F2hc subunit is known to translocate other light subunits to the plasma membrane for functional activity to occur (Chillaron et al, 2001; Verrey et al, 2004), suggesting that xCT subunits located in the plasma membrane of BxPC-3 cells are likely functional. The fact that prominent xCT protein localisation to the plasma membrane was not seen in the other cell lines tested may be explained by the observation that BxPC-3 cells morphologically grow in tighter groups of cells, facilitating the visualisation of xCT protein at cell–cell (intercellular) plasma membrane junctions. In contrast to BxPC-3 cells, DEM induced upregulation of xCT protein in MIA PaCa-2 and PANC-1 cells within the intracellular compartment (Figure 3D). Indeed, subcellular localisation studies of xCT in HEK cells have reported xCT expression not only at the plasma membrane, but also at intracellular membranes such as the lysosomal or endosomal membranes (Shih and Murphy, 2001). The functional significance of intracellular xCT expression, however, remains to be determined.
Although use of the oxidative stressor DEM was able to increase xCT expression, it did not have an effect on 4F2hc mRNA or protein expression (Figure 3B and C). The xCT promoter region contains an antioxidant response element (ARE) that regulates transcription of the gene (Wasserman and Fahl, 1997; Ishii et al, 2000). In contrast, the presence of an ARE has not been reported in the 4F2hc promoter region, suggesting a possible explanation for the observed difference in response to oxidative stress for the two genes. Alternatively, basal levels of 4F2hc may be much higher than those of xCT, potentially rendering changes in 4F2hc expression undetectable. Indeed, in a blood–retinal barrier cell line, mRNA levels of 4F2hc were reported to be 56-fold higher than those of xCT (Tomi et al, 2002). This suggests that 4F2hc mRNA in some cells is in excess, most likely because of the fact that 4F2hc is a common component of many other amino-acid transport systems.
Among normal tissues, xCT is expressed predominantly in normal pancreas (Bassi et al, 2001; Kim et al, 2001), specifically in the islet cell population (Bassi et al, 2001). Furthermore, xCT has also been reported to exhibit higher expression in an acinar cell line compared with a pancreatic islet cell line (Sato et al, 1998). The present study shows that xCT is expressed in normal pancreatic tissues preferentially in the ductal cells compared with the acinar cells. Importantly, in primary human pancreatic ductal adenocarcinoma specimens, xCT protein is overexpressed throughout the cancerous ductal structures (Figure 4). Given that the xc− transporter is overexpressed in pancreatic cancers, and that expression of the xc− transporter can be induced in pancreatic cancer cell lines in response to oxidative stress, our findings implicate the xc− transporter as an important mediator of pancreatic cancer cell proliferation and survival.
The higher xCT expression by the PANC-1 cells suggest that they have more xc− cystine transporters per cell than the other cells and hence a greater ability to take up cystine from the microenvironment, likely synthesising more GSH when needed. It has been reported that NIH3T3 cells treated with sublethal levels of DEM to induce ROS defense genes such as xCT and glutathione-S-transferase α1 (GSTα1) are associated with higher resistance to various apoptotic stimuli such as buthionine sulphoximine, ultraviolet, and etoposide (Faraonio et al, 2006). Moreover, HT22 hippocampal cells resistant to oxidative stress exhibited a 7-fold overexpression of xCT mRNA, with a concomitant increase in resistance to glutamate-induced oxidative stress and hydrogen peroxide-induced oxidative stress (Lewerenz et al, 2006). In the present study, although PANC-1 cells express a 3.5-fold higher level of xCT mRNA compared with the MIA PaCa-2 and BxPC-3 cell lines, PANC-1 cells are ∼1000 × more resistant to GEM. This suggests that GEM resistance cannot be solely explained by higher xCT mRNA expression, but rather that additional GEM resistance mechanisms are at play. Nonetheless, overexpressing xCT and 4F2hc in pancreatic cancer cells resulted in an overall increase in the GEM resistant phenotype (Figure 6). The fact that overexpressing the xc− transporter was able to increase cell survival even in the absence of GEM treatment highlights the importance of the xc− transporter as a regulator of pancreatic cancer cell proliferation and survival. Furthermore MSG, a potent and specific inhibitor of the xc− transporter (Bannai, 1986), significantly inhibited the growth of the pancreatic cancer cell lines. By including 2-ME (66 μ M) in the culture medium, which provides an alternative route for cystine uptake through L-transport system (Ishii et al, 1981), growth arrest was prevented. This gives direct evidence that the pancreatic cancer cell lines critically depend on the xc− transporter for growth and survival. Unfortunately, MSG cannot be used for therapy as it is neurotoxic (Choi, 1988).
Our results demonstrate that pancreatic cancer cells exhibit upregulated expression of the xc− transporter, which promotes uptake of extracellular cystine for synthesis of GSH. In turn, elevated intracellular GSH levels promote pancreatic cancer cell growth and survival in response to the production of reactive oxygen species by oxidative stressors, including the chemotherapeutic agent GEM. Hence, drug resistance in pancreatic cancer may be explained in part by overexpression of the xc− transporter. Finally, the marked growth arrest of the pancreatic cancer cell lines obtained through specific inhibition of the xc− transporter suggests that this transporter may serve as a target for therapy of the disease.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Babich H, Borenfreund E (1991) Cytotoxicity of T-2 toxin and its metabolites determined with the neutral red cell viability assay. Appl Environ Microbiol 57: 2101–2103
Bannai S (1984a) Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem 259: 2435–2440
Bannai S (1984b) Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779: 289–306
Bannai S (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 261: 2256–2263
Bassi MT, Gasol E, Manzoni M, Pineda M, Riboni M, Martin R, Zorzano A, Borsani G, Palacin M (2001) Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc. Pflugers Arch 442: 286–296
Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V (2001) Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42: 47–54
Brosnan JT, Brosnan ME (2006) The sulfur-containing amino acids: an overview. J Nutr 136: 1636S–1640S
Chawla RK, Lewis FW, Kutner MH, Bate DM, Roy RG, Rudman D (1984) Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology 87: 770–776
Chillaron J, Roca R, Valencia A, Zorzano A, Palacin M (2001) Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am J Physiol Renal Physiol 281: F995–F1018
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623–634
Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25: 7101–7110
Donadelli M, Costanzo C, Beghelli S, Scupoli MT, Dandrea M, Bonora A, Piacentini P, Budillon A, Caraglia M, Scarpa A, Palmieri M (2007) Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim Biophys Acta 1773: 1095–1106
Doxsee DW, Gout PW, Kurita T, Lo M, Buckley AR, Wang Y, Xue H, Karp CM, Cutz JC, Cunha GR, Wang YZ (2007) Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 67: 162–171
Estrela JM, Ortega A, Obrador E (2006) Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci 43: 143–181
Faraonio R, Vergara P, Marzo DD, Napolitano M, Russo T, Cimino F (2006) Transcription regulation in NIH3T3 cell clones resistant to diethylmaleate-induced oxidative stress and apoptosis. Antioxid Redox Signal 8: 365–374
Freelove R, Walling AD (2006) Pancreatic cancer: diagnosis and management. Am Fam Physician 73: 485–492
Gmunder H, Eck HP, Droge W (1991) Low membrane transport activity for cystine in resting and mitogenically stimulated human lymphocyte preparations and human T cell clones. Eur J Biochem 201: 113–117
Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME (1992) High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA 89: 3070–3074
Gout PW, Buckley AR, Simms CR, Bruchovsky N (2001) Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)- cystine transporter: a new action for an old drug. Leukemia 15: 1633–1640
Gout PW, Kang YJ, Buckley DJ, Bruchovsky N, Buckley AR (1997) Increased cystine uptake capability associated with malignant progression of Nb2 lymphoma cells. Leukemia 11: 1329–1337
Griffith OW (1999) Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med 27: 922–935
Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Saeki S, Kanai Y, Endou H, Naito M, Tsuruo T, Terasaki T (2002) Enhancement of L-cystine transport activity and its relation to xCT gene induction at the blood-brain barrier by diethyl maleate treatment. J Pharmacol Exp Ther 302: 225–231
Iglehart JK, York RM, Modest AP, Lazarus H, Livingston DM (1977) Cystine requirement of continuous human lymphoid cell lines of normal and leukemic origin. J Biol Chem 252: 7184–7191
Ishii T, Bannai S, Sugita Y (1981) Mechanism of growth stimulation of L1210 cells by 2-mercaptoethanol in vitro. Role of the mixed disulfide of 2-mercaptoethanol and cysteine. J Biol Chem 256: 12387–12392
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275: 16023–16029
Iwata S, Hori T, Sato N, Hirota K, Sasada T, Mitsui A, Hirakawa T, Yodoi J (1997) Adult T cell leukemia (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by L-cystine and glutathione depletion: possible involvement of thiol-mediated redox regulation in apoptosis caused by pro-oxidant state. J Immunol 158: 3108–3117
Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H (2001) Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 1512: 335–344
Lewerenz J, Klein M, Methner A (2006) Cooperative action of glutamate transporters and cystine/glutamate antiporter system Xc- protects from oxidative glutamate toxicity. J Neurochem 98: 916–925
Lo M, Wang YZ, Gout PW (2008) The x(c) (-) cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J Cell Physiol 215: 593–602
Maehara S, Tanaka S, Shimada M, Shirabe K, Saito Y, Takahashi K, Maehara Y (2004) Selenoprotein P, as a predictor for evaluating gemcitabine resistance in human pancreatic cancer cells. Int J Cancer 112: 184–189
McBean GJ, Flynn J (2001) Molecular mechanisms of cystine transport. Biochem Soc Trans 29: 717–722
Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR (2007) Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: enhancement of growth-inhibitory activity of doxorubicin. Chemotherapy 53: 210–217
Noda T, Iwakiri R, Fujimoto K, Rhoads CA, Aw TY (2002) Exogenous cysteine and cystine promote cell proliferation in CaCo-2 cells. Cell Prolif 35: 117–129
Rosado JO, Salvador M, Bonatto D (2007) Importance of the trans-sulfuration pathway in cancer prevention and promotion. Mol Cell Biochem 301: 1–12
Sato H, Kuriyama-Matsumura K, Siow RC, Ishii T, Bannai S, Mann GE (1998) Induction of cystine transport via system x-c and maintenance of intracellular glutathione levels in pancreatic acinar and islet cell lines. Biochim Biophys Acta 1414: 85–94
Sato H, Tamba M, Ishii T, Bannai S (1999) Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 274: 11455–11458
Schnelldorfer T, Gansauge S, Gansauge F, Schlosser S, Beger HG, Nussler AK (2000) Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 89: 1440–1447
Shih AY, Murphy TH (2001) xCt cystine transporter expression in HEK293 cells: pharmacology and localization. Biochem Biophys Res Commun 282: 1132–1137
Stabler SP, Lindenbaum J, Savage DG, Allen RH (1993) Elevation of serum cystathionine levels in patients with cobalamin and folate deficiency. Blood 81: 3404–3413
Tomi M, Hosoya K, Takanaga H, Ohtsuki S, Terasaki T (2002) Induction of xCT gene expression and L-cystine transport activity by diethyl maleate at the inner blood-retinal barrier. Invest Ophthalmol Vis Sci 43: 774–779
Uren JR, Lazarus H (1979) L-cyst(e)ine requirements of malignant cells and progress toward depletion therapy. Cancer Treat Rep 63: 1073–1079
Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 447: 532–542
Wasserman WW, Fahl WE (1997) Functional antioxidant responsive elements. Proc Natl Acad Sci USA 94: 5361–5366
Wolff RA (2007) Chemotherapy for pancreatic cancer: from metastatic disease to adjuvant therapy. Cancer J 13: 175–184
Zalatnai A, Molnar J (2007) Review. Molecular background of chemoresistance in pancreatic cancer. In Vivo 21: 339–347
Acknowledgements
M Lo held a Doctoral Research Award from the Canadian Institutes of Health Research. The work was supported by grants from the National Cancer Institute of Canada – Canadian Cancer Society (VL), the Canadian Institutes of Health Research (PWG/YZ) and the British Columbia Cancer Foundation (PWG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lo, M., Ling, V., Wang, Y. et al. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br J Cancer 99, 464–472 (2008). https://doi.org/10.1038/sj.bjc.6604485
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604485
Keywords
This article is cited by
-
Simplified and highly-reliable automated production of [18F]FSPG for clinical studies
EJNMMI Radiopharmacy and Chemistry (2023)
-
Prevotella histicola suppresses ferroptosis to mitigate ethanol-induced gastric mucosal lesions in mice
BMC Complementary Medicine and Therapies (2023)
-
Selenite as a dual apoptotic and ferroptotic agent synergizes with EGFR and KRAS inhibitors with epigenetic interference
Clinical Epigenetics (2023)
-
Exploring the role of the disulfidptosis-related gene SLC7A11 in adrenocortical carcinoma: implications for prognosis, immune infiltration, and therapeutic strategies
Cancer Cell International (2023)
-
Glutathione and glutathione disulfide – their biomedical and pharmaceutical applications
Medicinal Chemistry Research (2023)