Abstract
Among the 51 823 postmenopausal women in the Swedish Mammography Cohort, we investigated breast cancer risk in relation to the FFQ-based estimated lignan intake by oestrogen receptor (ER) and progesterone receptor (PR) subtypes. A significant 17% risk reduction for breast cancer overall in the high lignan quartile was observed, especially among PMH user (Pinteraction<0.010), but no heterogeneity across ER/PR subtypes.
Similar content being viewed by others
Main
Plant lignans, a major type of phytoestrogens in Nordic countries, are mainly present in cereals, fruit, and vegetables (Adlercreutz, 1998a, 1998b) and are metabolised to mammalian lignans (e.g. enterolactone (ENL)) by the intestinal microflora (Adlercreutz, 2002). Since a preventive action of lignans against breast cancer was suggested (Adlercreutz et al, 1982), this has been evaluated in vitro (Welshons et al, 1987; Hirano et al, 1990; Mousavi and Adlercreutz, 1992), in vivo (Serraino and Thompson, 1991, 1992) and in clinical studies (Adlercreutz et al, 1988, 1991; Phipps et al, 1993; Thompson et al, 2005). Biological plausibility was discussed in a recent review (Adlercreutz, 2007). Hormone-dependent (Adlercreutz et al, 1992, 1993) and other mechanisms (Hirano et al, 1990; Kitts et al, 1999; Mäkelä et al, 1999; Prasad, 2000; Rickard et al, 2000) have been suggested. Six prospective (den Tonkelaar et al, 2001; Keinan-Boker et al, 2004; Kilkkinen et al, 2004; Olsen et al, 2004; Touillaud et al, 2007; Verheus et al, 2007) and six case–control studies (Pietinen et al, 2001; Dai et al, 2002; McCann et al, 2002, 2004, 2006; Fink et al, 2007) have evaluated the issue among postmenopausal women. Of these, only four considered oestrogen and progesterone receptor status of tumours (ER/PR) (den Tonkelaar et al, 2001; Olsen et al, 2004; McCann et al, 2006; Touillaud et al, 2007). We therefore examined the issue in a large population-based cohort study with stratification by family history of breast cancer, level of alcohol intake, body mass index, and use of postmenopausal hormone (PMH).
Materials and methods
The Swedish Mammography Cohort (SMC) was described previously (Wolk et al, 1998; Suzuki et al, 2006). It was established in 1987–90 that all women in Västmanland who were born in 1917–48, and in Uppsala born in 1914–48, were invited. A total of 66 651 women completed a questionnaire including diet. In 1997, a second questionnaire was sent to all cohort members. We excluded those with missing or incorrect data, with previous cancer (except non-melanoma skin cancer), who were not post-menopausal and who were 70+ years old at baseline leaving a cohort of 51 823 women. The information on diet was collected through self-administrated food-frequency questionnaires in 1987 and 1997. Total lignan intake were estimated using published values of following four lignans; secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol (Mazur et al, 1996, 1998a, 1998b, 2000; Adlercreutz and Mazur, 1997; Mazur and Adlercreutz, 1998; Valsta et al, 2003; Milder et al, 2005; Penalvo et al, 2005; Schwartz and Sontag, 2006; Thompson et al, 2006). Other nutrients were calculated based on the Swedish National Food Administration database (Bergström et al, 1991). Cereals (60%), vegetables (27%), and fruits (10%) are the main sources of our lignans. Among a random sample of 137 women from the cohort, the correlation between the FFQ-based estimates of lignan intake and serum ENL levels measured by time-resolved fluoroimmunoassay (Adlercreutz et al, 1998) was r=0.2 (Spearman's rank). Date of breast cancer diagnosis, death, or migration from the study area were identified by linkage of the cohort through the Swedish Registration System. Information about receptor status of breast tumours, measured by an Abbott immunoassay (Pousette et al, 1986) and an immunohistochemical method, was obtained from Uppsala University Hospital and the Regional Oncology Centre. The study was approved by the Regional Ethics Committee at the Uppsala University Hospital and Karolinska Institute. We used time-dependent multivariate Cox proportional hazards regression model to estimate hazard rate ratios and 95% confidence intervals with age as the time scale (Korn et al, 1997). We subdivided lignan intakes into four categories based on approximate quartiles. Trend tests were conducted by using the median value for each category of lignans as a continuous variable. Heterogeneity in the results between the ER+PR+ and other subtypes was evaluated using the Wald statistic (Liao, 2004). P-value for interaction was evaluated by a likelihood ratio test. Analyses were performed by SAS system, version 9.1 (SAS Institute, Cary, NC, USA). Statistical tests were two-sided, and significance levels defined as P<0.05.
Results
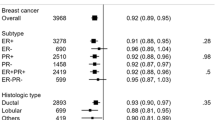
Among 51 823 women with an average 8.3-year follow-up, 1284 invasive breast cancer cases were diagnosed, with details of ER/PR status available for 1188 cases. Of these, 716 were ER+PR+, 279 ER+PR−, 50 ER−PR+, and 143 ER−PR− tumours. Women with high lignan intake tended to be older, have more education and have greater use of PMH (Table 1).
Overall, we observed a statistically significant inverse association between lignan intake and breast cancer risk (Table 2). Compared to women in the lowest quartile (<712 μg day−1), the multivariable adjusted relative risks (RR) for the highest quartile (⩾1036 μg day−1) were 0.83 (95% confidence interval=0.70–0.97; Ptrend=0.042) for overall, 0.86 (0.69–1.08) for ER+PR+, 0.77 (0.54–1.09) for ER+PR−, 0.92(0.56–1.52) for ER−PR−. There was no evidence for heterogeneity in the results between the ER+PR+ and other subtypes (all Pheterogeneities⩾0.65).
In the full adjusted analysis stratified by family history of breast cancer, by levels of alcohol intake and by body mass index (<25 or ⩾25 kg/m2), there was no evidence for interaction with lignans in relation to overall risk or of any subtype; all Pvalues for trends were>0.60 and all Pvalues for interaction>0.35. We also observed a significant inverse association of lignans with overall risk among PMH ever-users; the multivariable adjusted RR for the highest quartile of intake compared to the lowest was 46% lower (Ptrend=<0.0001; Table 3). In contrast, among PMH never-users, no association was observed (Pinteraction=0.01). The observed interaction for PMH use seemed to be confined to ER+PR+ tumors (Pinteraction=0.016). There was no heterogeneity in the results between ER+PR+ and other tumors (all Pheterogeneity ⩾0.21). Lignans were positively correlated with intake of fruits and vegetables (r=0.4) and of cereal, fruit and vegetable fibre (r=0.7, 0.2 and 0.4, respectively). After adjusting for these factors, the result for lignans was slightly attenuated but still significant among PMH user (Table 3).
Discussion
In this large population-based prospective cohort of postmenopausal women, we observed a significant inverse association between lignan intake and overall breast cancer risk, especially among PMH user. There was no evidence of heterogeneity across ER/PR tumours. These results are similar to our previous study with a significant inverse association between cereal fibre and breast cancer risk among PMH users (Suzuki et al, 2008). The estimated lignan intake was correlated with cereal fibre (r=0.7) but after adjusting for specific fibres, the association among PMH users was still significant. This inverse association agrees with two previous studies among postmenopausal women (Fink et al, 2007; Touillaud et al, 2007). Non-significant inverse associations (Pietinen et al, 2001; Dai et al, 2002; McCann et al, 2002, 2004; Keinan-Boker et al, 2004; Olsen et al, 2004; Verheus et al, 2007) and no association (den Tonkelaar et al, 2001; Kilkkinen et al, 2004; McCann et al, 2006) have also been reported.
An inverse association of lignans with risk has been reported among premenopausal women (Dai et al, 2002; McCann et al, 2002, 2004, 2006; Linseisen et al, 2004; Piller et al, 2006a), among women with palpable cysts (Boccardo et al, 2004), and high epidermal growth factor concentrations (Boccardo et al, 2003), and among those carrying the A2 allele of CYP17 (McCann et al, 2002; Piller et al, 2006b) possibly associated with increased levels of endogenous hormone (Haiman et al, 1999). Given these findings, an inverse relation of risk with lignans is probable in subgroups of women with high circulating oestrogen level just as discussed with regard to isoflavone (Glazier and Bowman, 2001). The possible biological mechanism is not clear, but in vitro studies also showed that lignan ENL in the presence of oestrogens suppressed the oestrogen-induced proliferation in MCF-7 breast cancer cell (Mousavi and Adlercreutz, 1992) and stimulated the synthesis of sex hormone-binding globulin in liver cells (Adlercreutz et al, 1992).
The lack of association among overweight women may be due to the relatively high circulating oestrogen levels from PMH use having a stronger effect than the endogenous oestrogens formed in peripheral tissues (Cleland et al, 1985; Jurgens et al, 1992; Hankinson et al, 1998). Compared to lean women, obese women tend to have a lower prevalence of PMH use (Suzuki et al, 2006) and lower level of plasma ENL (Kilkkinen et al, 2001; Johnsen et al, 2004). Body fat might attenuate the effect of lignans by suppressing intestinal microflora activity (Nishizawa et al, 1988), or trapping ENL (Johnsen et al, 2004).
Our finding for ER+PR+ tumours among PMH users partly agrees with a prospective study (Touillaud et al, 2007), though these results were not confined to PMH users. No association was reported in two prospective studies (den Tonkelaar et al, 2001; Olsen et al, 2004) and a case–control study (McCann et al, 2006). Some nutrient misclassification and individual variation in intestinal microflora, as well as the lack of detailed information about PMH use are all relevant. Lignan estimates were not highly correlated with plasma ENL, but the observed correlation was comparable to those reported previously (Kilkkinen et al, 2003; Hedelin et al, 2006). In prospective cohort design, this misclassification of exposure tends to be nondifferential which may attenuate the observed association toward null. Further studies need to elucidate this issue with taking the circulating level of oestrogens into consideration.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adlercreutz H (1998a) Epidemiology of Phytoestrogens. London: Bailliere Tindall
Adlercreutz H (1998b) Epidemiology of phytoestrogens. Baillieres Clin Endocrinol Metab 12: 605–623
Adlercreutz H (2002) Phyto-oestrogens and cancer. Lancet Oncol 3: 364–373
Adlercreutz H (2007) Lignans and human health. Crit Rev Clin Lab Sci 44: 483–525
Adlercreutz H, Bannwart C, Wähälä K, Mäkelä T, Brunow G, Hase T, Arosemena PJ, Kellis Jr JT, Vickery LE (1993) Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44: 147–153
Adlercreutz H, Fotsis T, Heikkinen R, Dwyer JT, Woods M, Goldin BR, Gorbach SL (1982) Excretion of the lignans enterolactone and enterodiol and of equol in omnivorous and vegetarian postmenopausal women and in women with breast cancer. Lancet 2: 1295–1299
Adlercreutz H, Höckerstedt K, Bannwart C, Hämäläinen E, Fotsis T, Bloigu S (1988) Association between dietary fiber, urinary excretion of lignans and isoflavonic phytoestrogens, and plasma non-protein bound sex hormones in relation to breast cancer. In Progress in Cancer Research and Therapy: Hormones and Cancer, Bresciani F, King RJB, Lippman ME, Raynaud J-P (eds) Vol. 35, 409–412. New York: Raven Press, Ltd
Adlercreutz H, Honjo H, Higashi A, Fotsis T, Hämäläinen E, Hasegawa T, Okada H (1991) Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am J Clin Nutr 54: 1093–1100
Adlercreutz H, Mazur W (1997) Phyto-oestrogens and Western diseases. Ann Med 29: 95–120
Adlercreutz H, Mousavi Y, Clark J, Höckerstedt K, Hämäläinen E, Wähäla K, Mäkelä T, Hase T (1992) Dietary phytoestrogens and cancer: in vitro and in vivo studies. J Steroid Biochem Mol Biol 41: 331–337
Adlercreutz H, Wang GJ, Lapcik O, Hampl R, Wähälä K, Mäkelä T, Lusa K, Talme M, Mikola H (1998) Time-resolved fluoroimmunoassay for plasma enterolactone. Anal Biochem 265: 208–215
Bergström LKE, Hagman U, Eriksson HB, Bruce Å (1991) The food composition database KOST: the National Food Administration's information system for nutritive values of food. Vår Föda 43: 439–447
Boccardo F, Lunardi G, Guglielmini P, Parodi M, Murialdo R, Schettini G, Rubagotti A (2004) Serum enterolactone levels and the risk of breast cancer in women with palpable cysts. Eur J Cancer 40: 84–89
Boccardo F, Lunardi GL, Petti AR, Rubagotti A (2003) Enterolactone in breast cyst fluid: correlation with EGF and breast cancer risk. Breast Cancer Res Treat 79: 17–23
Cleland WH, Mendelson CR, Simpson ER (1985) Effects of aging and obesity on aromatase activity of human adipose cells. J Clin Endocrinol Metab 60: 174–177
Dai Q, Franke AA, Jin F, Shu XO, Hebert JR, Custer LJ, Cheng J, Gao YT, Zheng W (2002) Urinary excretion of phytoestrogens and risk of breast cancer among Chinese women in Shanghai. Cancer Epidemiol Biomarkers Prev 11: 815–821
den Tonkelaar I, Keinan-Boker L, Veer PV, Arts CJ, Adlercreutz H, Thijssen JH, Peeters PH (2001) Urinary phytoestrogens and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 10: 223–228
Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD (2007) Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol 165: 514–523
Glazier MG, Bowman MA (2001) A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 161: 1161–1172
Haiman CA, Hankinson SE, Spiegelman D, Colditz GA, Willett WC, Speizer FE, Kelsey KT, Hunter DJ (1999) The relationship between a polymorphism in CYP17 with plasma hormone levels and breast cancer. Cancer Res 59: 1015–1020
Hankinson SE, Willett WC, Manson JE, Colditz GA, Hunter DJ, Spiegelman D, Barbieri RL, Speizer FE (1998) Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst 90: 1292–1299
Hedelin M, Klint A, Chang ET, Bellocco R, Johansson JE, Andersson SO, Heinonen SM, Adlercreutz H, Adami HO, Gronberg H, Balter KA (2006) Dietary phytoestrogen, serum enterolactone and risk of prostate cancer: the cancer prostate Sweden study (Sweden). Cancer Causes Control 17: 169–180
Hirano T, Fukuoka K, Oka K, Naito T, Hosaka K, Mitsuhashi H, Matsumoto Y (1990) Antiproliferative activity of mammalian lignan derivatives against the human breast carcinoma cell line, ZR-75-1. Cancer Invest 8: 595–602
Johnsen NF, Hausner H, Olsen A, Tetens I, Christensen J, Knudsen KE, Overvad K, Tjonneland A (2004) Intake of whole grains and vegetables determines the plasma enterolactone concentration of Danish women. J Nutr 134: 2691–2697
Jurgens Jr RW, Downey LJ, Abernethy WD, Cutler NR, Conrad J (1992) A comparison of circulating hormone levels in postmenopausal women receiving hormone replacement therapy. Am J Obstet Gynecol 167: 459–460
Keinan-Boker L, van Der Schouw YT, Grobbee DE, Peeters PH (2004) Dietary phytoestrogens and breast cancer risk. Am J Clin Nutr 79: 282–288
Kilkkinen A, Stumpf K, Pietinen P, Valsta LM, Tapanainen H, Adlercreutz H (2001) Determinants of serum enterolactone concentration. Am J Clin Nutr 73: 1094–1100
Kilkkinen A, Valsta LM, Virtamo J, Stumpf K, Adlercreutz H, Pietinen P (2003) Intake of lignans is associated with serum enterolactone concentration in Finnish men and women. J Nutr 133: 1830–1833
Kilkkinen A, Virtamo J, Vartiainen E, Sankila R, Virtanen MJ, Adlercreutz H, Pietinen P (2004) Serum enterolactone concentration is not associated with breast cancer risk in a nested case-control study. Int J Cancer 108: 277–280
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU (1999) Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 202: 91–100
Korn EL, Graubard BI, Midthune D (1997) Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145: 72–80
Liao TF (2004) Comparing social groups: Wald Statistics for testing equality among Multiple Logit Models. Int J Comparative Sociology 45: 3–16
Linseisen J, Piller R, Hermann S, Chang-Claude J (2004) Dietary phytoestrogen intake and premenopausal breast cancer risk in a German case–control study. Int J Cancer 110: 284–290
Mäkelä S, Strauss L, Saarinen N, Salmi S, Streng T, Joshi S, Santti R (1999) Dietary phytoestrogens—mechanisms of action and possible role in the development of hormonally dependent diseases. In Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease, Kumpulainen JT, Salonen JT (eds) pp 349–355. London: Royal Society of Chemistry
Mazur W, Adlercreutz H (1998) Naturally occurring oestrogens in food. Pure & Appl Chem 70: 1759–1776
Mazur W, Fotsis T, Wähälä K, Ojala S, Salakka A, Adlercreutz H (1996) Isotope dilution gas chromatographic-mass spectrometric method for the determination of isoflavonoids, coumestrol, and lignans in food samples. Anal Biochem 233: 169–180
Mazur WM, Duke JA, Wähälä K, Rasku S, Adlercreutz H (1998a) Isoflavonoids and lignans in legumes: nutritional and health aspects in humans. J Nutr Biochem 9: 193–200
Mazur WM, Uehara M, Wähälä K, Adlercreutz H (2000) Phyto-oestrogen content of berries, and plasma concentrations and urinary excretion of enterolactone after a single strawberry-meal in human subjects. Br J Nutr 83: 381–387
Mazur WM, Wähälä K, Rasku S, Salakka A, Hase T, Adlercreutz H (1998b) Lignan and isoflavonoid concentrations in tea and coffee. Br J Nutr 79: 37–45
McCann SE, Kulkarni S, Trevisan M, Vito D, Nie J, Edge SB, Muti P, Freudenheim JL (2006) Dietary lignan intakes and risk of breast cancer by tumor estrogen receptor status. Breast Cancer Res Treat 99: 309–311
McCann SE, Moysich KB, Freudenheim JL, Ambrosone CB, Shields PG (2002) The risk of breast cancer associated with dietary lignans differs by CYP17 genotype in women. J Nutr 132: 3036–3041
McCann SE, Muti P, Vito D, Edge SB, Trevisan M, Freudenheim JL (2004) Dietary lignan intakes and risk of pre- and postmenopausal breast cancer. Int J Cancer 111: 440–443
Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (2005) Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr 93: 393–402
Mousavi Y, Adlercreutz H (1992) Enterolactone and estradiol inhibit each other's proliferative effect on MCF-7 breast cancer cells in culture. J Steroid Biochem Mol Biol 41: 615–619
Nishizawa Y, Imaizumi T, Tanishita H, Yano I, Kawai Y, Mormii H (1988) Relationship of fat deposition and intestinal microflora in VMH rats. Int J Obes 12: 103–110
Olsen A, Knudsen KE, Thomsen BL, Loft S, Stripp C, Overvad K, Moller S, Tjonneland A (2004) Plasma enterolactone and breast cancer incidence by estrogen receptor status. Cancer Epidemiol Biomarkers Prev 13: 2084–2089
Penalvo JL, Haajanen KM, Botting N, Adlercreutz H (2005) Quantification of lignans in food using isotope dilution gas chromatography/mass spectrometry. J Agric Food Chem 53: 9342–9347
Phipps WR, Martini MC, Lampe JW, Slavin JL, Kurzer MS (1993) Effect of flax seed ingestion on the menstrual cycle. J Clin Endocrinol Metab 77: 1215–1219
Pietinen P, Stumpf K, Männistö S, Kataja V, Uusitupa M, Adlercreutz H (2001) Serum enterolactone and risk of breast cancer: a case-control study in eastern Finland. Cancer Epidemiol Biomarkers Prev 10: 339–344
Piller R, Chang-Claude J, Linseisen J (2006a) Plasma enterolactone and genistein and the risk of premenopausal breast cancer. Eur J Cancer Prev 15: 225–232
Piller R, Verla-Tebit E, Wang-Gohrke S, Linseisen J, Chang-Claude J (2006b) CYP17 genotype modifies the association between lignan supply and premenopausal breast cancer risk in humans. J Nutr 136: 1596–1603
Pousette A, Gustafsson SA, Thornblad AM, Nordgren A, Sallstrom J, Lindgren A, Sundelin P, Gustafsson JA (1986) Quantitation of estrogen receptor in seventy-five specimens of breast cancer: comparison between an immunoassay (Abbott ER-EIA monoclonal) and a [3H]estradiol binding assay based on isoelectric focusing in polyacrylamide gel. Cancer Res 46: 4308s–4309s
Prasad K (2000) Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, enterodiol, and enterolactone. Int J Angiol 9: 220–225
Rickard SE, Yuan YV, Thompson LU (2000) Plasma insulin-like growth factor I levels in rats are reduced by dietary supplementation of flaxseed or its lignan secoisolariciresinol diglycoside. Cancer Lett 161: 47–55
Schwartz H, Sontag G (2006) Determination of secoisolariciresinol, lariciresinol and isolariciresinol in plant foods by high performance liquid chromatography coupled with coulometric electrode array detection. J Chromatogr B Analyt Technol Biomed Life Sci 838: 78–85
Serraino M, Thompson LU (1991) The effect of flaxseed supplementation on early risk markers for mammary carcinogenesis. Cancer Lett 60: 135–142
Serraino M, Thompson LU (1992) The effect of flaxseed supplementation on the initiation and promotional stages of mammary tumorigenesis. Nutr Cancer 17: 153–159
Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Adlercreutz H, Wolk A (2008) Dietary fiber intake and risk of postmenopausal breast cancer defined by estrogen and progesterone receptor status-A prospective cohort study among Swedish women. Int J Cancer 122: 403–412
Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Wolk A (2006) Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: A Prospective Cohort Study. Int J Cancer 119: 1683–1689
Thompson LU, Boucher BA, Liu Z, Cotterchio M, Kreiger N (2006) Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr Cancer 54: 184–201
Thompson LU, Chen JM, Li T, Strasser-Weippl K, Goss PE (2005) Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res 11: 3828–3835
Touillaud MS, Thiebaut AC, Fournier A, Niravong M, Boutron-Ruault MC, Clavel-Chapelon F (2007) Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J Natl Cancer Inst 99: 475–486
Valsta LM, Kilkkinen A, Mazur W, Nurmi T, Lampi AM, Ovaskainen ML, Korhonen T, Adlercreutz H, Pietinen P (2003) Phyto-oestrogen database of foods and average intake in Finland. Br J Nutr 89 (Suppl 1): S31–S38
Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH (2007) Plasma phytoestrogens and subsequent breast cancer risk. J Clin Oncol 25: 648–655
Welshons WV, Murphy CS, Koch R, Calaf G, Jordan VC (1987) Stimulation of breast cancer cells in vitro by the environmental estrogen enterolactone and the phytoestrogen equol. Breast Cancer Res Treat 10: 169–175
Wolk A, Bergstrom R, Hunter D, Willett W, Ljung H, Holmberg L, Bergkvist L, Bruce A, Adami HO (1998) A prospective study of association of monounsaturated fat and other types of fat with risk of breast cancer. Arch Intern Med 158: 41–45
Acknowledgements
This study was funded by research grants from the Swedish Cancer Foundation and the Swedish Research Council/Longitudinal studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Suzuki, R., Rylander-Rudqvist, T., Saji, S. et al. Dietary lignans and postmenopausal breast cancer risk by oestrogen receptor status: a prospective cohort study of Swedish women. Br J Cancer 98, 636–640 (2008). https://doi.org/10.1038/sj.bjc.6604175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604175
Keywords
This article is cited by
-
Lignan exposure: a worldwide perspective
European Journal of Nutrition (2022)
-
Dietary lignan intake and androgen receptor expression in breast tumors
Cancer Causes & Control (2015)
-
Oilseeds ameliorate metabolic parameters in male mice, while contained lignans inhibit 3T3-L1 adipocyte differentiation in vitro
European Journal of Nutrition (2014)
-
Consumption of flaxseed, a rich source of lignans, is associated with reduced breast cancer risk
Cancer Causes & Control (2013)
-
Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study
Breast Cancer Research and Treatment (2013)