Abstract
When chemotherapy is used in androgen-independent prostate cancer (AIPC), androgen deprivation is continued despite its failure. In this study, we investigated whether it was possible to re-induce hormone sensitivity in previously castrate patients by stopping endocrine therapy during chemotherapy. A phase II prospective study investigated the effects of reintroduction of endocrine therapy after oral chemotherapy in 56 patients with AIPC, which was given without concurrent androgen deprivation. After chemotherapy, patients were given maximum androgen blockade until failure when treatment was switched to diethylstilbestrol and dexamethasone. Patients had already received these endocrine treatments in the same sequence before chemotherapy. All patients were castrate at the start of chemotherapy. Forty-three subsequently restarted endocrine therapy after the completion of chemotherapy. The median overall survival for these 43 patients from the time of restarting endocrine therapy was 7.7 months (95% confidence interval (CI): 3.7–10.9 months). Sixteen (37%) patients had a 50% PSA response to treatment, which was associated with improved overall survival (14.0 months vs 3.7 months P=0.003). Eight out of 12 patients who did not respond to diethylstilbestrol before chemotherapy did so post chemotherapy. Re-induction of hormone sensitivity can occur after chemotherapy in AIPC.
Similar content being viewed by others
Main
There is an increasing number treatment options for androgen-independent prostate cancer (AIPC). Responses have been seen to steroids and estrogens (Farrugia et al, 2000; Nishimura et al, 2000), and chemotherapy has shown a survival advantage for these patients (Petrylak et al, 2004; Tannock et al, 2004). Until recently, it has been a universal practice to continue androgen deprivation during chemotherapy, despite previous progression on this treatment. Obviously, stopping treatment could result in restoration of androgen levels and growth of androgen-sensitive clones. However preclinical data suggest that chemotherapy in AIPC cell lines is more effective if given sequentially rather than concurrently (Tang et al, 2006). Additionally, in breast cancer, sequential hormone therapy and chemotherapy might be advantageous (Cavalli et al, 1983; Kim et al, 2005).
In a previous chemotherapy study involving castrate patients AIPC, we anecdotally observed that re-induction of hormone sensitivity was possible if endocrine therapy was stopped during chemotherapy (Shamash et al, 2005). Therefore, a prospective phase II study was designed to investigate this observation.
Materials and methods
Between May 2003 and January 2006, castrate patients (testosterone <1.5 nmol l−1) with AIPC, who had in addition also failed diethylstilbestrol (1 mg) and dexamethasone (2 mg), were recruited into this study. All patients received oral chemotherapy with chlorambucil 1 mg kg−1 starting day 1, lomustine 2 mg kg−1 day 1, etoposide 50 mg daily from day 22–28 and 36–42, with the cycle repeating every 56 days. This treatment was based on a previously published regimen (Shamash et al, 2005). Patients were biochemically castrate (testosterone <1.5 nmol l−1 or failed maximum androgen blockade (MAB)) before starting the chemotherapy. All endocrine therapy was stopped during chemotherapy.
The entry criteria required for this study included histologic verification of prostate cancer, prostate-specific antigen (PSA) progression of disease despite failure of androgen deprivation and subsequent oestrogen (diethylstilbestrol 1 mg at least or equivalent) and corticosteroid (dexamethasone 2 mg or equivalent). This study was approved by the ethics committee and patients gave written informed consent before therapy.
Following failure of chemotherapy, patients recommenced endocrine therapy, initially with MAB (bicalutamide and GnRH analogue), and when this failed, patients were given dexamethasone 2 mg daily and diethylstilbestrol 1 mg daily (DS). Patients were seen monthly with PSA measurement, clinical and toxicity assessment.
In this study, progression of disease was based on PSA, using the Prostate-Specific Antigen Working Group criteria published in 1999 throughout (Bubley et al, 1999).
Thirteen of the 56 chemotherapy patients in this study did not restart endocrine therapy at the time of progression on chemotherapy. Twelve of these patients died of progression of disease before reintroduction of endocrine therapy could occur, and one refused to go back on endocrine therapy.
The log-rank test was used to look at which categorical variables were significant on univariate analysis for overall survival at the time of reintroduction of hormone therapy. Multivariate analysis was performed by fitting a Cox proportional hazard model to the data.
The Mann–Whitney two-sample statistic was used to compare median values between groups. Frequency tables and proportions were examined using Fisher's exact test.
Results
Forty-three patients with AIPC went on to receive further endocrine therapy after disease progression on chemotherapy. The characteristics of these patients are shown in Table 1, Figure 1.
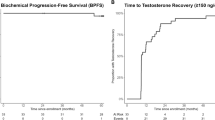
The median overall survival for these patients, from the time of restarting endocrine therapy, was 7.7 months (95% CI: 3.7–10.9 months).
Twenty-two patients (51%) were non-castrate (testosterone >1.5 nmol l−1) at the start of endocrine therapy. Median overall survival for the non-castrate patients was 8.7 months (95% CI: 3.3–20.0) vs 6.4 months (95% CI: 2.6–12.1) for the castrate patients (P=0.33).
Sixteen of the 43 patients (37%) had a PSA response to treatment (either MAB or DS, which was associated with better overall survival compared with those with no PSA response (14.0 months (95% CI: 6.3–26.4) vs 3.7 months (95% CI: 2.1–8.7): P=0.003).
The median PSA progression free survival on MAB after chemotherapy was 1.7 months (range: 0.3–9.7 months). Eight (19%) patients had a PSA response to MAB, and these responders were on treatment for a median of 3.9 months (range 0.4–6.8). Two of 17 (12%) biochemically castrate patients had a PSA response to MAB, while six of 22 (27%) non-castrate patients had a PSA response to MAB (P=0.43).
Twenty-eight patients (65%) of the cohort went on to subsequently receive DS after MAB. The remainder did not receive DS because of contraindications to treatment or rapid disease progression on MAB. The median survival from the time of starting DS was 9.8 months (95% CI: 5.5–16.4). Twelve (43%) of these patients had a PSA response. Obtaining a PSA response to DS was associated with improved survival (median overall survival 16.5 months (95%CI: 6.9 to infinity) vs 5.5 months (95% CI: 2.7–13.3) (P=0.02). Overall, four patients responded to both MAB and DS.
The response to DS was independent of whether or not the patients were castrate at the end of chemotherapy (4/13 vs 8/14 (P=0.25)). Eight out of 12 patients who did not respond to DS before chemotherapy subsequently responded when rechallenged.
In multivariate analysis, the established prognostic factors (Gleason score, PSA at diagnosis, presence of bone metastasis) were unable to predict the likelihood of further hormone response following failure of chemotherapy.
Discussion
These prospective data confirm our anecdotal observation and suggest that endocrine sensitivity can be reintroduced by stopping endocrine therapy during chemotherapy for AIPC. Therefore, stopping endocrine therapy during chemotherapy may not be harmful, especially for the subset of patients who respond to it a second time. Randomised studies are required to answer this question formally.
It is intriguing that a group of patients should respond to endocrine therapy for a second time. This includes biochemically castrate and non-castrate patients, which suggests that when progression of disease occurs during chemotherapy, in the absence of concurrent androgen deprivation, both hormone-dependent and -independent clones may be responsible for the tumour regrowth. Therefore, it appears that chemotherapy may alter the subsequent behaviour of the disease. This theory is supported by the observation that some patients responded to DS after but not before chemotherapy. Additionally, patients who remained biochemically castrate after chemotherapy were less likely to respond to MAB than those who were not. Nevertheless, a large proportion of this group responded to DS, which was the treatment they received immediately before chemotherapy.
One of the possible confounding factors of this work is that patients responding to endocrine therapy a second time may have more indolent tumours and the PSA response is irrelevant. However, the PSA doubling time before chemotherapy did not correlate with a subsequent response to endocrine therapy (52 vs 57 days (P=0.60)).
There are a number of postulated mechanisms for the development of androgen independence in prostate cancer, including mutations of the androgen receptor to allowing other molecules to bind to it (Grossmann et al, 2001). There are in vitro data to suggest that this may be the case with diethylstilbestrol (Kalach et al, 2005). It may be that the prostate cancer cells, which regrow during chemotherapy in the presence of androgen ablation therapy, have molecular characteristics different from those for which chemotherapy is given alone. It is even possible that removing hormonal suppression during chemotherapy may allow cells to enter the cell cycle and become more sensitive to cytotoxic drugs.
A shortcoming of this work was that it did not use docetaxel chemotherapy, which has become the standard treatment for these patients. However, the case report published in this edition of the British Journal of Cancer suggests this may be possible with docetaxel and requires further investigation.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17(11): 3461–3467
Cavalli F, Beer M, Martz G, Jungi WF, Alberto P, Obrecht JP, Mermillod B, Brunner KW (1983) Concurrent or sequential use of cytotoxic chemotherapy and hormone treatment in advanced breast cancer: report of the Swiss Group for Clinical Cancer Research. Br Med J (Clin Res Ed) 286(6358): 5–8
Farrugia D, Ansell W, Singh M, Philp T, Chinegwundoh F, Oliver RT (2000) Stilboestrol plus adrenal suppression as salvage treatment for patients failing treatment with luteinizing hormone-releasing hormone analogues and orchidectomy. BJU Int 85(9): 1069–1073
Grossmann ME, Huang H, Tindall DJ (2001) Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst 93(22): 1687–1697. Review
Kalach JJ, Joly-Pharaboz MO, Chantepie J, Nicolas B, Descotes F, Mauduit C, Benahmed M, André J (2005) Divergent biological effects of estradiol and diethylstilbestrol in the prostate cancer cell line MOP. J Steroid Biochem Mol Biol 96(2): 119–129
Kim R, Tanabe K, Emi M, Uchida Y, Osaki A, Toge T (2005) Rationale for sequential tamoxifen and anticancer drugs in adjuvant setting for patients with node- and receptor-positive breast cancer. Int J Oncol 26(4): 1025–1031
Nishimura K, Nonomura N, Yasunaga Y (2000) Low doses of oral dexamethasone for hormone refractory prostate carcinoma. Cancer 2570–2576
Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer [see comment]. N Engl J Med 351(15): 1513–1520
Shamash J, Barlow C, Wilson P, Ansell W, Oliver RTD (2005) Chlorambucil and lomustine (CL56) in absolute hormone refractory prostate cancer: re-induction of endocrine sensitivity, an unexpected finding. Br J Cancer 92(1): 36–40
Tang Y, Khan MA, Goloubeva O, Lee DI, Jelovac D, Brodie AM, Hussain A (2006) Docetaxel followed by castration improves outcomes in LNCaP prostate cancer-bearing severe combined immunodeficient mice. Clin Cancer Res; 9(15):5550-8 12(1): 169–174
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX 327 Investigators (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer [see comment]. N Engl J Med 351(15): 1502–1512
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shamash, J., Davies, A., Ansell, W. et al. A phase II study investigating the re-induction of endocrine sensitivity following chemotherapy in androgen-independent prostate cancer. Br J Cancer 98, 22–24 (2008). https://doi.org/10.1038/sj.bjc.6604051
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6604051
Keywords
This article is cited by
-
Wirkmechanismen der fortgesetzten und unterbrochenen LHRH-Therapie
Uro-News (2020)
-
Importance of androgen-deprivation therapy during enzalutamide treatment in men with metastatic castration-resistant prostate cancer following chemotherapy: results from retrospective, multicenter data
Prostate Cancer and Prostatic Diseases (2019)
-
Androgen deprivation therapy in castrate-resistant prostate cancer: how important is GnRH agonist backbone therapy?
World Journal of Urology (2015)
-
Comment on ‘Anti-tumour activity of abiraterone and diethylstilboestrol when administered sequentially to men with castration-resistant prostate cancer’
British Journal of Cancer (2014)
-
Reply: ‘Comment on Anti-tumour activity of abiraterone and diethylstilboestrol when administered sequentially to men with castration-resistant prostate cancer’
British Journal of Cancer (2014)