Abstract
High expression of thymidylate synthase (TS) and inactivation of p53 are allegedly associated with chemoresistance. The authors evaluated TS and p53 expression in gastric cancer treated with neoadjuvant S-1/cisplatin chemotherapy. Paraffin sections of pretreatment biopsy and surgical specimens from 41 gastric cancers were immunostained for TS and p53 protein after appropriate antigen retrieval. Fifty-one cases without neoadjuvant chemotherapy were also studied. In the pretreatment biopsies, high expression of TS was seen in 8% of the histologic responders, in 28% of the nonresponders and in 31% of the controls. High expression of p53 was observed in 56% of the nonresponders, but in 8% of the responders and in 29% of the controls (P<0.01 and P<0.05, respectively). The TS- and/or p53-high phenotype was seen in 76% of the nonresponders and in 54% of the controls, but in 8% of the responders (P<0.0001 and P<0.005, respectively). The data of the surgical specimens were consistent with those of the pretreatment biopsies. These results suggest that immunostaining for TS and p53 protein is useful for pretreatment selection of gastric cancer patients unresponsive to S-1/cisplatin chemotherapy.
Similar content being viewed by others
Main
Gastric cancer is declining in incidence, but it remains a significant cause of mortality (Parkin et al, 1988). Resectability is one of the most important prognostic factors in treating gastric cancer (Yeh and Cheng, 2004). Even in resectable tumours, the recurrence rate is significantly high. For downstaging and lowering the rate of postoperative recurrence, neoadjuvant chemotherapy for gastric cancer has been performed in the high-risk group (Yonemura et al, 1996; Yeh and Cheng, 2004). It has been reported that histological grading is a useful prognostic indicator in gastric cancer treated with neoadjuvant chemotherapy (Yonemura et al, 1996).
Fluoropyrimidines (5-fluorouracil (5-FU) and its prodrugs) are most widely used for the treatment of solid tumours including gastric cancer. Several kinds of fluoropyrimidines are now in use. S-1 is a novel fluoropyrimidine consisting of tegafur, 5-chloro-2, 4-dihydroxypyrimidine (CDHP, a strong inhibitor of dihydropyrimidine dehydrogenase, 5-FU-catabolizing enzyme) and potassium oxonate with a molar ratio of 1 : 0.4 : 1 (Malet-Martino and Martino, 2002). S-1, especially in combination with cisplatin, shows a promising effectiveness with acceptable toxicities against gastric, pancreatic and non-small-cell lung cancers (Schoffski, 2004; Yoshida et al, 2005).
Analysis of the predictors for identifying patients who will be sensitive or resistant to chemotherapeutic agents is one of the approaches for individualizing the treatment. The appropriate decision for chemotherapy leads to the avoidance of unpleasant side effects. At the present time, however, there are no clinically accepted markers that can accurately predict the sensitivity or resistance of gastric cancer against chemotherapeutic agents.
Thymidylate synthase (TS) is the target enzyme of 5-FU, and the inhibition of TS activity is the principal mechanism of its cytotoxicity. Plural preclinical studies have suggested that high TS levels are closely associated with the 5-FU resistance of cancer cells (Beck et al, 1994; Peters et al, 1994). Investigators have reported the clinical relevance of high TS mRNA as a predictor of the resistance to 5-FU/cisplatin or S-1 in gastric cancer (Lenz et al, 1996; Ichikawa et al, 2004). However, the relationship between immunohistochemical expression of TS in pretreatment biopsy specimens of gastric cancer and the response to fluoropyrimidine-based chemotherapy has revealed conflicting results. Some investigators have shown that the TS expression in gastric cancer is of limited value in predicting the clinical response to fluoropyrimidine-based chemotherapy (Boku et al, 1998; Miyamoto et al, 2000). In contrast, there is a report demonstrating the clinical relevance of high TS expression as a predictor of the resistance to fluoropyrimidine-based chemotherapy and the short survival of the patients (Yeh et al, 1998).
The product of the tumour-suppressor gene, p53, is a pleiotropic molecule with plurifunctions. p53 protein normally functions as either a repair initiator of the damaged sense DNA or a trigger of the apoptotic pathway if a sufficient level of damage takes place within the cell (Leonard et al, 1995; Harwood et al, 1996). It has been demonstrated that p53-dependent apoptosis is associated with the cytotoxic effects of cisplatin or 5-FU plus cisplatin for gastric cancer cells (Ikeguchi et al, 1997; Satomi et al, 2002; Matsuhashi et al, 2004). The absence of wild-type p53 function results in the cellular resistance to anticancer agents such as 5-FU and cisplatin in several cell lines, including gastric cancer (Nabeya et al, 1995; Harris, 1996). In addition, several investigators have demonstrated the correlation between the immunohistochemical overexpression of p53 in primary gastric cancer and the resistance to cisplatin-contained regimens (Hamada et al, 1996; Cascinu et al, 1998; Nakata et al, 1998).
There is evidence that TS forms a ribonucleoprotein complex with p53 mRNA (Liu et al, 2002). It is thereby suggested that TS may play a critical role in regulating the cell cycle and the process of apoptosis through its regulatory effects on the expression of p53. In the present study, we immunohistochemically analysed the relationship between the expression of TS and p53 and the effects of neoadjuvant S-1/cisplatin chemotherapy for gastric cancers. We demonstrate here that TS- and/or p53-high expression can be a relevant and useful predictor in determining S-1/cisplatin resistance of gastric cancer.
Materials and methods
Patients and specimens
Table 1 summarizes pretreatment clinicopathological features of 92 patients included in the present study. All the patients were treated for advanced gastric cancer between 2001 and 2006 at the Department of Surgery, Fujita Health University Hospital, Toyoake, Japan. Forty-one patients were preoperatively treated with the combination of S-1 (80–120 mg m−2 per day for 4 weeks) and cisplatin (35 mg m−2 on day 8). One cycle of this regimen was completed in 6 weeks and the next cycle of this regimen was started after 2 weeks off of S-1 administration since the last day of the S-1 administration. At least two cycles of this regimen were performed, depending on the clinical status of these patients. The median of this regimen was consequently two cycles. We have adopted the dose of 35 mg m−2 on day 8 of cisplatin, because of the fact that S-1 alone and/or S-1 plus low-dose cisplatin must have caused a significant clinical effect. We have experienced cases showing a significant clinical effect after S-1 alone and/or S-1 plus low-dose cisplatin in neoadjuvant settings (Yoshida et al, 2005). Fifty-one patients who did not receive neoadjuvant chemotherapy were also analysed as controls. The two groups were closely matched and there were no differences in the clinicopathological backgrounds. The present study was approved by the institutional ethical review board for human investigation at Fujita Health University.
Pretreatment biopsy specimens and surgically resected tumours were routinely fixed in 10% formalin and embedded in paraffin wax. Sections, 3 μm thick, were cut and mounted on aminopropyltriethoxysilane slides, and stained with haematoxylin and eosin (HE), in order to assess histopathological features and the responsiveness to neoadjuvant chemotherapy under a light microscope.
Histological assessment of chemotherapeutic effects
Chemotherapeutic effects were histologically evaluated using the surgical specimens, according to the Japanese Classification of Gastric Carcinoma (Japanese Research Society for Gastric Cancer, 1999). Major grading (grades 0–3) and additional minor grading for grade 1 (grades 1a and b) were used, based upon the degree of necrosis or disappearance of tumour cells in the lesion; grade 0: no change, grade 1: mild change (grade 1a: necrosis or disappearance of the tumour seen in less than 1/3 of the entire cancer area, which is regarded as undistinguishable from spontaneous tumour necrosis; and grade 1b: necrosis or disappearance of the tumour seen in more than 1/3 but less than 2/3 of the entire cancer area), grade 2: moderate change (necrosis or disappearance of the tumour seen in more than 2/3 of the entire cancer area, but still with remaining viable tumour cells) and grade 3: severe change (no viable tumour cells remain). The patients with grades 1b and 2 were categorised as the histologic responders, and the patients with grades 0 and 1a as the histologic nonresponders. No grade 3 case was observed.
Immunohistochemistry
In addition to pretreatment biopsy specimens, one or two representative paraffin blocks of the resected tumour were chosen for immunohistochemical analysis. The sections were deparaffinised with xylene and rehydrated with graded ethanols. Endogenous peroxidase was inactivated by 0.03% hydrogen peroxide in methanol for 30 min. Heat-induced epitope retrieval was applied using a pressure cooker (Delicio 6L; T-FAL, Rumily, France) for 10 min. Optimal soaking solutions, determined by preliminary experiments (Kamoshida et al, 2003a, 2003b), were selected for the respective markers: 1 mM ethylenediaminetetraacetic acid solution, pH 8.0 for TS and 10 mM citrate buffer, pH 7.0 for p53. After pressure-cooking, the sections were left at room temperature for cooling in the soaking solution for 30 min.
The sections were then incubated with the primary antibody that reacts specifically with TS (rabbit polyclonal, 1 : 200 dilution; Taiho Pharmaceutical Co., Tokushima, Japan) or p53 (mouse monoclonal, clone DO-7, 1 : 200 dilution; Dako Co., CA, USA), overnight at room temperature. Histofine Simple Stain MAX-PO (Nichirei, Tokyo, Japan), employing the universal immunoperoxidase polymer method, was utilised as a second-layer reagent. The reaction products were visualised in 50 mg dl−1 3,3′-diaminobenzidine tetrahydrochloride solution containing 0.003% hydrogen peroxide. The nuclei were lightly counterstained with Mayer's haematoxylin. Negative control studies were performed without applying the primary antibodies. Sections known to be stained positively were included in each run as positive staining controls.
Evaluation of immunostaining
The HE-stained and immunostained sections were independently reviewed by two investigators (SK and MS), without prior knowledge of clinical data of the patients. Thymidylate synthase expression was classified into two groups in a semiquantitative manner: ‘low’ expression (negative or positive in 50% or less of tumour cells) vs ‘high’ expression (positive in more than 50% of tumour cells). Most of the other investigators defined TS expression of 25–30% as a threshold level (Yeh et al, 1998; Miyamoto et al, 2000). The reason for the difference is explained as follows: (1) to the best of our belief, TS staining method employed in our study is very sensitive, suitable and reproducible (Kamoshida et al, 2003a); (2) in our previous studies using colorectal cancer specimens, 30% was defined as a threshold level of TS expression (Kamoshida et al, 2003b, 2004). It has been reported that the mean TS mRNA level in gastric cancers is 1.6- to 1.7-fold higher than that in colorectal cancers, and the TS mRNA level is closely correlated with immunohistochemical expression of TS (Johnston et al, 1995; Uchida et al, 2001).
The pattern of immunohistochemical expression of p53 protein, the proportion of p53-positive cells, must be very important in discussing the relationship between p53 protein expression and gene mutation (Hall and Lane, 1994). The occurrence of occasional positive cells does not seem to correlate with obvious abnormality of p53 gene, but the positive staining in the majority of cells is frequently associated with gene mutation (Baas et al, 1994; Shiao et al, 2000). For p53, thus, negative or positive staining in 70% or less of tumour cells was considered ‘low’ expression, and positive staining in more than 70% of tumour cells was ‘high’ expression. According to the principle of imunohistochemical evaluation using multiple clinical samples, the intensity of staining should not be considered for the judgement of positivity (Kamoshida et al, 2004).
The evaluation of biopsy specimens was regarded appropriate when two or more biopsy pieces were available, as reported previously (Kamoshida et al, 2003b).
Statistical analysis
The Fisher's exact probability test was employed for determining the statistical significance of correlations between the marker expression and histological chemotherapeutic effects. P-values <0.05 were considered statistically significant.
Results
Marker expression
Thymidylate synthase immunoreactivity was observed in the cytoplasm of cancer cells. p53 protein was invariably localized in the nuclei. Little difference in the staining pattern of TS and p53 was seen between invasive and noninvasive components within the same tumours. Epithelial cells located in the generative zone of normal gastric mucosa and in the basal half of intestinal metaplastic mucosa were consistently immunoreactive for TS. In addition, TS was expressed in such nonepithelial cells as germinal centre lymphocytes, plasma cells, endothelial cells, fibroblasts and smooth muscle cells. No apparent p53 expression was detected in the non-neoplastic tissue and cells.
Chemotherapeutic effects and marker expression in pretreatment biopsy specimens
Histological chemotherapeutic responders consisted of 15 (37%) out of 41 cases (grade 1b: six cases and grade 2: nine cases), and the remaining 26 (63%) were categorised as the nonresponders (grade 0: eight cases and grade 1a: 18 cases).
Table 2 shows the relationship between chemotherapeutic effects and marker expression in the pretreatment biopsy specimens. Adequate biopsy material obtained from two cancerous parts or more was available in 38 out of the 41 cases receiving neoadjuvant chemotherapy and in 48 out of the 51 control untreated cases. High TS expression was observed in one lesion (8%) of 13 responders, in seven lesions (28%) of 25 nonresponders and in 15 lesions (31%) of 48 control untreated cases: no significant differences were noted.
High expression of p53 was observed in 14 lesions (56%) of the nonresponders and in 14 lesions (29%) of the control cases, but in one lesion (8%) of the responders: there was significant difference between the responders and the nonresponders (P<0.01), and between the nonresponders and the control cases (P<0.05). Accuracy of the p53 expression for predicting chemoresistance was 68%; 26 (12 responders and 14 nonresponders) out of 38 patients treated with neoadjuvant chemotherapy.
The TS- and/or p53-high phenotype was demonstrated in 19 lesions (76%) of the nonresponders and 26 lesions (54%) of the control cases, but in one lesion (8%) of the responders: the differences between the responders and the nonresponders or the control cases were statistically significant (P<0.0001 and P<0.005, respectively). Accuracy of the combination of TS and p53 expression for predicting chemoresistance was 82%; 31 (12 responders and 19 nonresponders) out of 38 patients treated with neoadjuvant chemotherapy. Representative immunostaining patterns in the pretreatment biopsy specimens are shown in Figures 1 and 2.
The pretreatment biopsy specimen (A–C) and the resected tumour (D) from a representative gastric cancer, which responded to neoadjuvant S-1/cisplatin chemotherapy. (A, D): HE staining, (B): TS immunoreactivity, (C): p53 immunoreactivity. Chemotherapy-induced histological changes including the disintegration of glandular structures with marked inflammatory cell infiltration is noted in the resected tumour when compared with the pretreatment biopsy specimen. The cytoplasm of only a few cancer cells (arrow) and plasma cells (arrowhead) in the stroma are positive for TS. There are no p53-positive cells.
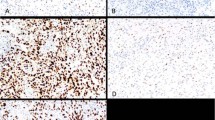
The pretreatment biopsy specimen (A–C, E–G) and the resected tumour (D, H) from two representative gastric cancers, which did not respond to neoadjuvant S-1/cisplatin chemotherapy. (A, D, E, H): Haematoxylin and eosin staining, (B, F): TS immunoreactivity, (C, G): p53 immunoreactivity. Little histological changes are discernible after chemotherapy. In a case shown in the top panels (A–D), a number of cancer cells express both TS and p53. In another case shown in the bottom panels (E–H), TS is expressed in a number of cancer cells. p53-positive nuclei are heterogeneously observed. Plasma cells in the stroma (arrows) are also immunoreactive for TS.
Chemotherapeutic effects and marker expression in surgically resected tumours
Table 3 shows the relationship between chemotherapeutic effects and marker expression in the surgical specimens. The data of the surgical specimens were consistent with those of the pretreatment biopsies. High expression of TS was seen in one lesion (7%) of 15 responders, in seven lesions (27%) of 26 nonresponders and in 14 lesions (27%) of 51 control untreated cases: no significant differences were detected. High expression of p53 was detected in 14 lesions (54%) of the nonresponders, but in one lesion (7%) of the responders and in 15 lesions (29%) of the control cases: The statistical significance was noted between the responders and the nonresponders (P<0.005), and between the nonresponders and the control cases (P<0.05). The TS- and/or p53-high phenotype was observed in 19 lesions (73%) of the nonresponders and 26 lesions (51%) of the control cases, but in one lesion (7%) of the responders: The differences between the responders and the nonresponders or the control cases were statistically significant (P<0.0001 and P<0.005, respectively).
Correlation of TS and p53 expression between pretreatment biopsy specimens and surgically resected materials
Table 4 demonstrates the concordance rate of TS and p53 expression between the pretreatment biopsy specimens and the resected materials. Thymidylate synthase expression was concordant in 34 (89%) out of 38 cases treated with neoadjuvant chemotherapy and in 41 (85%) out of 48 control untreated cases. The concordance of p53 expression was noted in 37 (97%) of the cases treated with neoadjuvant chemotherapy and in all of the control cases.
Discussion
To our knowledge, the present study is an initial attempt to predict the effects of S-1/cisplatin chemotherapy by the combination of TS and p53 immunostaining in gastric cancer. Thymidylate synthase, a critical target for fluoropyrimidines, catalyses the methylation of deoxyuridine monophosphate to deoxythymidine monophosphate, an essential step in DNA biosynthesis (Malet-Martino and Martino, 2002). When the pretreatment biopsy specimens were analysed, high expression of TS was observed in 8% of the responders and in 28% of the nonresponders, without significant difference. Yeh et al (1998) reported that high expression of TS significantly predicted the resistance to high dose 5-FU and leucovorin and the short survival of the patients, but others failed to demonstrate the significant relationship between the TS expression and the effects of fluoropyrimidine-based chemotherapy (Boku et al, 1998; Miyamoto et al, 2000). These results suggest that the effects of fluoropyrimidine-based chemotherapy for gastric cancers are unable to be predicted by TS alone.
p53 participates in apoptotic pathways following treatment with DNA-damaging agents such as cisplatin (Ikeguchi et al, 1997; Satomi et al, 2002; Matsuhashi et al, 2004), and inactivation of the p53 gene contributes to the resistance to anticancer agents in several cell lines, including gastric cancer (Nabeya et al, 1995; Harris, 1996). Fifteen years ago, a series of important issues were raised concerning the immunohistochemical assessment of p53 protein (Wynford-Thomas, 1992). Of particular note was that immunohistochemically detected p53 protein expression did not necessarily indicate the presence of p53 genetic mutations. The pattern of immunohistochemical expression of p53 protein, the proportion of p53-positive cells, should be crucial in discussing the relationship between p53 immunoreactivity and genetic mutation (Hall and Lane, 1994). For example, focal (heterogeneous) expression, the occurrence of dispersed positive cells, does not seem to correlate with the obvious abnormality of p53 gene (Baas et al, 1994; Shiao et al, 2000). It may rather reflect an accumulation of wild-type p53 protein as a result of either a response to DNA damage, alterations in the normal degradation process, or the stabilization of the gene product by an interaction with viral or cellular proteins (Baas et al, 1994; Kirsch and Kastan 1998). In contrast, diffuse (homogeneous) expression, the positive staining in the majority of cells, is frequently associated with mutation (Baas et al, 1994; Shiao et al, 2000). This view is supported by the clinical data, in which the differences in expression patterns are significantly correlated with the patient's prognosis (Barnes et al, 1993; Shiao et al, 2000). In addition, the intensity of staining should not be considered for the judgment of staining results, according to the principle of immunohistochemical evaluation using multiple clinical samples (Kamoshida et al, 2004). Still, it remains possible that there might be some discordance between p53 immunoreactivity and the genetic abnormality: for example, the presence of missing deletions may result in the abolishment of protein production, and some point mutations may lead to the production of unstable proteins without an increase of the half-life (Wynford-Thomas, 1992).
In the present study, p53 expression was classified into two groups: low expression (negative or nuclear positivity in ⩽70% of tumour cells) vs high expression (nuclear positivity in >70% of tumour cells). High expression of p53 in the pretreatment biopsy specimen was observed in 56% of the nonresponders but in 8% of the responders (P<0.01). These results let us postulate that the high expression of p53 protein in our series may reflect the diffuse (homogenous) overexpression of the mutant-type protein, leading to the resistance to S-1/cisplatin chemotherapy. However, this must be confirmed by additional study using molecular assays, such as single-strand conformational polymorphism analysis and direct sequencing.
When we analysed the combination of TS and p53 expression in the pretreatment biopsy, TS- and/or p53-high phenotype was seen in 76% of the nonresponders but in 8% of the responders (P<0.0001). Accuracy predicting the chemoresistance to S-1/cisplatin was 82% when p53 expression was combined with TS expression, whereas it was 68% when only p53 expression was applied. These results indicate that the TS- and/or p53-high phenotype as determined by immunohistochemistry is a strong predictor of the resistance to S-1/cisplatin chemotherapy. Reportedly, TS forms a ribonucleoprotein complex with the p53 mRNA and the functional consequence of the binding is translational repression (Liu et al, 2002). In the lesions showing p53-low but TS-high phenotype (observed in five lesions of the nonresponders but in none of the responders), thus, TS may play a critical role in regulating the process of apoptosis through its regulatory effects on expression of p53.
We demonstrated acceptable consistency in TS and p53 expression between the pretreatment biopsy specimens and surgically resected materials. The results suggest that the expression of TS and p53 protein is hardly changed after S-1/cisplatin chemotherapy, and immunostaining of TS and p53 in pretreatment biopsy specimens can be utilised for predicting the chemoresistance to S-1/cisplatin. Discrepant results of TS were probably due to the heterogenous distribution seen in most cancer tissues. In vitro study has demonstrated that twice the amount of TS is observed in gastric cancer cells after continuous exposure to 5-FU, when compared to the untreated cells (Yukimoto et al, 2001). However, the TS induction after drug exposure has not been documented in gastric cancer patients undergoing fluoropyrimidine-based chemotherapy (Uchida et al, 2001). A similar discrepancy between in vitro and in vivo systems has found in colorectal cancer (Peters et al, 2000; Uchida et al, 2001; Kamoshida et al, 2003b).
In conclusion, we demonstrated that high expression of TS and/or p53 in the pretreatment biopsy specimens predicted the chemoresistance to S-1 plus cisplatin in gastric cancer. We believe that the data of the present retrospective study provide with the basis for a prospective study, in which immunohistochemical expression of TS and p53 is utilised as a weapon for tailor-made chemotherapy.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baas IO, Mulder JWR, Offerhaus JA, Vogelstein B, Hamilton SR (1994) An evaluation of siz antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol 172: 5–12
Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR (1993) Immunohistological detection of p53 in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol 24: 469–476
Beck A, Etienne MC, Chéradame S, Fischel JL, Formento P, Renée N, Milano G (1994) A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer 30A: 1517–1522
Boku N, Chin K, Hosokawa K, Ohtsu A, Tajiri H, Yoshida S, Yamao T, Kondo H, Shirao K, Shimada Y, Saito D, Hasebe T, Mukai K, Seki S, Saito H, Johnston PG (1998) Biological markers as a predictor for response and prognosis of unresectable gastric cancer patients treated with 5-fluorouracil and cisplatinum. Clin Cancer Res 4: 1469–1474
Cascinu S, Graziano F, Ferro ED, Staccioli MP, Ligi M, Carnevali A, Muretto P, Catalano G (1998) Expression of p53 protein and resistance to preoperative chemotherapy in locally advanced gastric carcinoma. Cancer 83: 1917–1922
Hall PA, Lane DP (1994) p53 in tumour pathology: can we trust immunohistochemistry? Revisited!. J Pathol 172: 1–4
Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N, Takahashi K, Roth JA, Tanaka N, Orita K (1996) The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol 122: 360–365
Harris CC (1996) Structure and function of the p53 tumor suppressor gene: clues for rational cancer therapeutic strategies. J Natl Cancer Inst 88: 1442–1455
Harwood FG, Frazier MW, Krajewski S, Reed JC, Houghton JA (1996) Acute and delayed apoptosis induced by thymidine deprivation correlates with expression of p53 and p53-regulated genes in colon carcinoma cells. Oncogene 12: 2057–2067
Ichikawa W, Takahashi T, Suto K, Yamashita T, Nihei Z, Shirota Y, Shimizu M, Sasaki Y, Hirayama R (2004) Thymidylate synthase predictive power is overcome by irinotecan combination therapy with S-1 for gastric cancer. Br J Cancer 91: 1245–1250
Ikeguchi M, Tatebe S, Kaibara N, Ito H (1997) Changes in levels of expression of p53 and the product of the bcl-2 in lines of gastric cancer cells during cisplatin-induced apoptosis. Eur Surg Res 29: 396–402
Japanese Research Society for Gastric Cancer (1999) Japanese Classification of Gastric Carcinoma, 1st English edn, pp 101–104. Tokyo: Kanehara Shuppan
Johnston PG, Lenz H-J, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L (1995) Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res 55: 1407–1412
Kamoshida S, Matsuoka H, Matsuyama A, Shimomura R, Maruta M, Tsutsumi Y (2003a) Reproducible and reliable immunohistochemical demonstration of thymidylate synthase in formalin-fixed, paraffin-embedded sections: Application of antigen retrieval in EDTA solution. Acta Histochem Cytochem 36: 115–118
Kamoshida S, Matsuoka H, Matsuyama A, Shimomura R, Maruta M, Tsutsumi Y (2003b) Immunohistochemical demonstration of thymidylate synthase (TS), p53 protein and bcl-XL protein in colorectal cancer with preoperative peroral chemotherapy: TS as marker of unresponsiveness to 5-fluorouracil. Ann Cancer Res Ther 11: 73–94
Kamoshida S, Matsuoka H, Ishikawa T, Maeda K, Shimomura R, Inada K, Tsutsumi Y (2004) Immunohistochemical evaluation of thymidylate synthase (TS) and p16INK4a in advanced colorectal cancer: implication of TS expression in 5-FU-based adjuvant chemotherapy. Jpn J Clin Oncol 34: 594–601
Kirsch DG, Kastan MB (1998) Tumor-suppressor p53: implication for tumor development and prognosis. J Clin Oncol 16: 3158–3168
Lenz H-J, Leichman CG, Danenberg KD, Danenberg PV, Groshen S, Cohen H, Laine L, Crookes P, Silberman H, Baranda J, Garcia Y, Li J, Leichman L (1996) Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol 14: 176–182
Leonard CJ, Canman CE, Kastan MB (1995) The role of p53 in cell-cycle control and apoptosis: Implications for cancer. In Important Advances in Oncology, DeVita V, Hellman S, Rosenberg SA (eds), pp 33–42. Philadelphia: J.B. Lippincott
Liu J, Schmitz JC, Lin X, Tai N, Yan W, Farrell M, Bailly M, Chen T, Chu E (2002) Thymidylate synthase as a translational regulator of cellular gene expression. Biochim Biophys Acta 1587: 174–182
Malet-Martino M, Martino R (2002) Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist 7: 288–323
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y, Saji S (2004) Expression of p53 protein as a predictor of the response to 5-fluorouracil and cisplatin chemotherapy in human gastrointestinal cancer cell lines evaluated with apoptosis by use of thin layer collagen gel. Int J Oncol 24: 807–813
Miyamoto S, Boku N, Ohtsu A, Yoshida S, Ochiai A, Okabe H, Fukushima M (2000) Clinical implications of immunoreactivity of thymidylate synthase and dihydropyrimidine dehydrogenase in gastric cancer treated with oral fluoropyrimidine (S-1). Study Group of S-1 for Gastric Cancer. Int J Oncol 17: 653–658
Nabeya Y, Loganzo Jr F, Maslak P, Lai L, de Oliveira AR, Schwarts GK, Blundell ML, Altorki NK, Kelsen DP, Albino AP (1995) The mutational status of p53 protein in gastric and esophageal adenocarcinoma cell lines predicts sensitivity to chemotherapeutic agents. Int J Cancer 64: 37–46
Nakata B, Chung KH, Ogawa M, Ogawa Y, Yanagawa K, Muguruma K, Inoue T, Yamashita Y, Onoda N, Maeda K, Sawada T, Sowa M (1998) p53 protein overexpression as a predictor of the response to chemotherapy in gastric cancer. Surg Today 28: 595–598
Parkin DM, Laara E, Muir CS (1988) Estimates of the worldwide frequency of sixteen majors cancers in 1980. Int J Cancer 41: 184–187
Peters GJ, van der Wilt CL, van Groeningen CJ (1994) Predictive value of thymidylate synthase and dihydropyrimidine dehydrogenase. Eur J Cancer 30A: 1408–1411
Peters GJ, van Triest B, Backus HH, Kuiper CM, van der Wilt CL, Pinedo HM (2000) Molecular downstream events and induction of thymidylate synthase in mutant and wild-type p53 colon cancer cell lines after treatment with 5-fluorouracil and the thymidylate synthase inhibitor raltitrexed. Eur J Cancer 36: 916–924
Satomi D, Takiguchi N, Koda K, Oda K, Suzuki H, Yasutomi J, Ishikura H, Miyazaki M (2002) Apoptosis and apoptosis-associated gene products related to the response to neoadjuvant chemotherapy for gastric cancer. Int J Oncol 20: 1167–1171
Schoffski P (2004) The modulated oral fluoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs 15: 85–106
Shiao Y-H, Palli D, Caporaso NE, Alvord WG, Amorosi A, Nesi G, Saieva C, Masala G, Fraumeni Jr JF, Rice JM (2000) Genetic and immunohistochemical analyses of p53 independently predict regional metastasis of gastric cancers. Cancer Epidemiol Biomarkers Prev 9: 631–633
Uchida K, Hayashi K, Kuramochi H, Takasaki K (2001) Changes in intratumoral thymidylate synthase (TS) and dihydropyrimidine dehydrogenase (DPD) mRNA expression in colorectal and gastric cancer during continuous tegafur infusion. Int J Oncol 19: 341–346
Wynford-Thomas D (1992) p53 in tumour pathology: can we trust immunocytochemistry? J Pathol 166: 329–330
Yeh KH, Cheng AL (2004) Recent advances in therapy for gastric cancer. J Formos Med Assoc 103: 171–185
Yeh KH, Shun CT, Chen CL, Lin JT, Lee WJ, Lee PH, Chen YC, Cheng AL (1998) High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer 82: 1626–1631
Yonemura Y, Kinoshita K, Fujimura T, Fushida S, Sawa T, Matsuki N, Tanaka S, Kamata T, Takashima T, Miyazaki I (1996) Correlation of the histological effects and survival after neoadjuvant chemotherapy on gastric cancer patients. Hepatogastroenterology 43: 1260–1272
Yoshida I, Sakurai Y, Komori Y, Tonomura S, Masui T, Shoji M, Nakamura Y, Imazu H, Uyama I, Ochiai M (2005) Successful downstaging by S-1-based chemotherapy followed by surgical resections for gastric carcinoma with extensive distant lymph node metastasis. Report of two cases and a review of cases with surgical resection after downstaging by S-1-based chemotherapy. Hepatogastroenterology 52: 978–984
Yukimoto K, Nakata B, Muguruma K, Yashiro M, Ohira M, Ishikawa T, Hino M, Hirakawa K (2001) Apoptosis and thymidylate synthase inductions by 5-fluorouracil in gastric cancer cells with or without p53 mutation. Int J Oncol 19: 373–378
Acknowledgements
This work was in part supported by a Grant-in-Aid for Scientific Research (no. 17590321) from the Japan Society for Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Kamoshida, S., Suzuki, M., Shimomura, R. et al. Immunostaining of thymidylate synthase and p53 for predicting chemoresistance to S-1/cisplatin in gastric cancer. Br J Cancer 96, 277–283 (2007). https://doi.org/10.1038/sj.bjc.6603546
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603546
Keywords
This article is cited by
-
FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression
Scientific Reports (2019)
-
Phase II trial of S-1 plus leucovorin in patients with advanced gastric cancer and clinical prediction by S-1 pharmacogenetic pathway
Cancer Chemotherapy and Pharmacology (2017)
-
Upregulation of periostin prevents P53-mediated apoptosis in SGC-7901 gastric cancer cells
Molecular Biology Reports (2013)
-
Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy
British Journal of Cancer (2010)
-
Prediction of clinical outcome of S-1-based chemotherapy for gastric cancer patients
Gastric Cancer (2009)