Abstract
It has been suggested that neuroleptic medication may decrease cancer risk. We compared cancer risks in a population-based cohort study of 25 264 users (⩾2 prescriptions) of neuroleptic medications in the county of North Jutland, Denmark, during 1989–2002, with that of county residents who did not receive such prescriptions. Statistical analyses were based on age-standardisation and Poisson regression analysis, adjusting for age, calendar period, COPD, liver cirrhosis or alcoholism, use of NSAID, and, for breast cancer, additionally for use of hormone therapy, age at first birth, and number of children. Use of neuroleptic medications was associated with a decreased risk for rectal cancer in both women and men (adjusted IRRs of 0.61 (95% confidence interval, 0.41–0.91) and 0.82 (0.56–1.19), respectively) and for colon cancer in female users (0.78; 0.62–0.98). Some risk reduction was seen for prostate cancer (0.87; 0.69–1.08), but breast cancer risk was close to unity (0.93; 0.74–1.17). Overall, treatment with neuroleptic medications was not related to a reduced risk of cancer, but for cancers of the rectum, colon and prostate there were suggestive decreases in risk.
Similar content being viewed by others
Main
Neuroleptic medications, in the form of chlorpromazine and other phenothiazine compounds, were introduced into clinical psychiatry in the 1940s and 1950s, and it was proposed early on that these medications might be associated with a reduced cancer risk (Jones, 1985). A recent review concluded that phenothiazines seem to have antiproliferative effects on many tumour cell lines in vitro correlating with an antagonistic effect on calmodulin activity, suggesting that calmodulin could be the intracellular target of phenothiazine-induced cell growth inhibition (Hait and Lazo, 1986; Nordenberg et al, 1999).
Neuroleptics have been hypothesised to account for the reduced cancer occurrence observed in patients with schizophrenia in a number of studies (Dupont et al, 1986; Mortensen, 1989; Gulbinat et al, 1992; Mortensen, 1994; Lichtermann et al, 2001; Dalton et al, 2005). This reduction has been found primarily in men in smoking-related cancers, and in prostate and rectal cancer. Except for a nested case–control study of prostate cancer among Danish schizophrenic in-patients that showed a reduced risk after treatment with chlorpromazine or other high-dose phenothiazines (Mortensen, 1992), the cohort studies of cancer incidence in schizophrenic patients have not included information on use of specific medications.
By contrast, concern has been raised that neuroleptic medication may increase breast cancer risk due to their prolactin-increasing effect (Schyve et al, 1978) and this has been reported among women with schizophrenia (Nakane and Ohta, 1986; Gulbinat et al, 1992; Lichtermann et al, 2001) and a range of psychiatric disorders (Halbreich et al, 1996; Wang et al, 2002). The few studies of breast cancer risk in patients treated with neuroleptics have mainly found no excess (Overall, 1978; Wagner and Mantel, 1978; Kanhouwa et al, 1984; Kelly et al, 1999), an exception being a recent cohort study (Wang et al, 2002).
We examined risks for cancer overall and for specific sites among adult users of neuroleptic medication in a population-based cohort study of all residents in a Danish county during a 14-year period.
Materials and methods
The study was conducted within the population of North Jutland, a Danish county with 500 000 inhabitants. The Danish National Health Service provides tax supported health care for all inhabitants, guaranteeing free access to general practitioners and hospitals, and refunds parts of the costs of drugs prescribed by physicians (Olivarius et al, 1997). All services are registered by use of the personal identification number, which is assigned to all Danish citizens by the Central Population Register (CPR) and encodes gender and date of birth. Use of the personal identification number secures valid linkage between population-based registries and allows for the establishment of complete cancer and prescription histories for each individual.
Study population
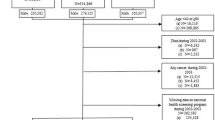
From the files of the CPR, we identified all 463 232 inhabitants of North Jutland County on 1 January 1989, who were 16–85 years of age during the period 1 January 1989–31 December 2002. We excluded 14 249 individuals with a history of cancer (except nonmelanoma skin cancer) prior to study entry (1989 or age 16 years) by linkage to the nation-wide Danish Cancer Registry, leaving 448 983 persons for follow-up. The Danish Cancer Registry has recorded incident cases of cancer on a nation-wide basis since 1943 and has been shown to have accurate and virtually complete ascertainment of cancer cases (Storm et al, 1997).
Drug exposure data
The North Jutland Prescription Database, established in 1989, holds key information about all prescriptions for refundable drugs dispensed from pharmacies in the county (Gaist et al, 1997; Nielsen et al, 1997). The data include type of drug prescribed according to the Anatomical Therapeutical Chemical (ATC) classification system, date of dispensing at the pharmacy, and the patient's personal identification number. Using the Prescription Database, we identified 25 264 individuals in the study population who received at least two prescriptions for neuroleptic medication (ATC code N05A) between 1 January 1989 and 31 December 2002 and who were free of cancer at date of second prescription. Persons with only one prescription for neuroleptic medication (N=10 877) were not included in this study. ATC code N05A covers the main neuroleptic categories, including phenothiazines, thiozanthenes, butyrophenones, diazepin-oxazepines, benzamides, diphenylbutylpiperidines and indol.
Psychiatric diagnosis data
The Psychiatric Case Register is a national register that has been computerised since 1 April 1969 and contains data on all admissions to Danish psychiatric in-patient facilities and, since 1995, information from outpatient contacts (Munk-Joergensen and Mortensen, 1997). There is no fee for psychiatric treatment in Denmark, and no private psychiatric facilities exist. The diagnostic system used during the study period was the International Classification of Diseases, eighth revision (ICD-8) up to 1993 and 10th revision (ICD-10) thereafter. Using the files of the Psychiatric Case Register, we identified all admissions for psychiatric disorders in the study population from 1969 through 2001. Patients with any hospitalisation or outpatient contact for schizophrenia were identified by ICD-8 code 295 and ICD-10 codes F20 and F25. All other psychiatric in- or outpatients were grouped together.
Data on other potential confounders
The county Hospital Discharge Registry (HDR) was established in 1977 and contains information on all hospitalisations in the county, including hospital and department number, dates of admission and discharge, and up to 20 diagnoses per hospitalisation and up to six operations per diagnosis (Andersen et al, 1999). Diagnoses are coded according to a Danish five-digit version of ICD-8 and ICD-10. We obtained information on all hospitalisations for chronic obstructive pulmonary disorder (COPD) (ICD-8, 490-492; ICD-10, J40-44), liver cirrhosis (ICD-8, 571,573; ICD-10, K70, K72, K74, K76) and alcoholism (ICD-8, 303; ICD-10, F10) between 1978 and 2002, as proxy measures for smoking habits and alcohol consumption that might confound the relationship between use of neuroleptic medications and cancer risk.
From the North Jutland Prescription Database, we further obtained prescription data for medications that potentially could confound the association between neuroleptic medication and cancer risk, including nonsteroidal anti-inflammatory drugs (NSAIDs) (ATC codes; M01A; N02BA01; N02BA51, and B01AC06) and hormone therapy (HT) (ATC groups: G03A-D and G03F).
For a subcohort, we obtained information on age at first childbirth and parity through linkage with the CPR. This information was available for all women born after 1935 and alive in 1968 or later. This restricted the base population in this analysis to 159 942 women and the exposed cohort to 6693 women.
Follow-up for cancer
Using information from the Cancer Registry and CPR, all individuals in the study population were followed through 31 December 2002 for any first primary diagnosis of cancer from 1 January 1989 or age 16 years, whichever occurred later, and continued until date of cancer diagnosis (except nonmelanoma skin cancer), death, emigration from North Jutland, or 31 December 2002, whichever came first. The person-time of the study subjects was distributed according to use of neuroleptic medication in exposed time (⩾2 prescriptions for neuroleptic medication) and unexposed time (no recorded prescriptions for neuroleptic medication). Person-time between first and second prescription for neuroleptic medication was excluded from the analyses. The exposed time was stratified into number of prescriptions (2–4, 5–9, 10–19, and 20 or more prescriptions).
Statistical analyses
We computed age- and gender-standardised incidence rates for the exposed group (neuroleptic medication users) by applying direct standardisation in gender-specific 10-year age groups to the age distribution of the entire study population (base population). Age- and gender-standardised incidence rate ratios (IRR) were calculated by dividing cancer incidence rates for neuroleptic users by cancer incidence rates for nonusers of neuroleptic medications (never users). Log-linear Poisson regression analysis, with the logarithm of person-years at risk as an offset variable, was used to compute rate ratios adjusted for calendar period (1989–1994, 1995–1999, 2000–2002), age (16–29, 30–39, 40–49, 50–59, 60–69, 70–84 years), hospitalisations for COPD, liver cirrhosis or alcoholism, and ever use of NSAID. Further, for the subcohort analysis of female breast cancer risk, the rate ratios were adjusted for ever use of HT and for number of children and age at first birth as linear covariates.
Stratified analyses were performed according to sex, number of prescriptions, years of follow-up and psychiatric disorders. Subjects were allowed to change between categories of covariates and exposure variables over time. Within each categorical level all variables were treated as time independent. A test for linear trend was used to evaluate dose response with number of prescriptions and years of follow-up. The statistical analyses were performed in SAS 8.02.
Results
Characteristics of the cohort of 25 264 neuroleptic users are presented in Table 1. In total, the users of neuroleptic medication accrued 161 478 person-years with a mean follow-up of 6.4 years (range 0–14 years). Compared with female users, more male users were diagnosed with schizophrenia and had a diagnosis of COPD or alcoholism. About a third of the exposed group received 20 or more prescriptions for neuroleptic medication during the study period. The cohort of neuroleptic users overall had a high frequency of psychiatric in- or outpatient contacts, with 6% being hospitalised with schizophrenia and 31% with other psychiatric diagnosis, as well as NSAID use (69%) and morbidity associated with heavy smoking (11%) and alcohol use (9%).
The mean age of the subcohort of 6693 women included in the analysis of breast cancer risk was 43.3 years at entry and mean follow-up was 7.8 years (data not shown). Among these female neuroleptic users, 8.5% had a diagnosis of schizophrenia and a further 33.2% had been hospitalised with another psychiatric disorder (data not shown).
The age- and gender-standardised incidence rate of cancer overall was 626 per 100 000 person-years among neuroleptic users and 543 per 100 000 person-years among never users, yielding an incidence rate ratio (IRR) of 1.15 (95% CI, 1.10–1.21) (Table 2). An increased IRR was observed for lung cancer in both male and female users, whereas a decreased risk was observed for rectal cancer and colon cancer in female users and for rectal cancer in male users (although these latter two risk estimates failed to reach statistical significance). For prostate and breast cancer, age-standardised IRRs were close to unity, however, when restricting the analysis to persons below 67 years of age the IRR for breast cancer went from 1.07 (95% CI, 0.95–1.21) to 0.92 (95% CI, 0.73–1.14) (Table 2). Adjustment for calendar period, diagnoses of COPD, liver cirrhosis, alcoholism, and use of NSAIDs reduced the risk for total cancer and most dramatically for lung cancer in both men and women. The IRR for prostate cancer was also reduced (IRR, 0.87; 95% CI, 0.69–1.08) but the risk estimates for colon or rectal cancer did not change materially; however, the reduced risk for colon cancer in female users reached statistical significance (Table 2). When stratified by type of psychiatric disorder, the rate ratios for prostate cancer and rectal cancer were similarly reduced among patients with schizophrenia, patients with other psychiatric disorders and never hospitalised users of neuroleptic medication (data not shown). For breast cancer, adjustment for parity, age at first birth and use of HT did not change the risk estimate in the subcohort aged 16–66 years. Excluding the first year of follow-up after second prescription for neuroleptic medication yielded similar overall results (data not shown).
There were no significant trends in the adjusted rate ratios by neither number of prescriptions or years of follow-up for cancers of the lung, rectum, colon, prostate or breast, respectively, although the risk estimates were generally increased (lung cancer) or reduced (rectal, colon, prostate and breast cancer) across all strata (data not shown). In persons with more than 10 prescriptions and more than 5 years of follow-up, a low rate ratio was observed for rectal cancer (IRR, 0.47; 95% CI, 0.18–1.26 in men and IRR, 0.33; 95% CI, 0.12–0.88 in women) and for prostate cancer (IRR, 0.66; 95% CI, 0.40–1.10) (data not shown).
Discussion
We found a reduced risk of rectal cancer in both men and women as well as indications of a reduced risk of colon cancer and prostate cancer in this population-based cohort of neuroleptic users, thus providing some support to the hypothesis of an anticarcinogenic effect of these medications. Reassuringly, we observed no increased risk for breast cancer in female users.
The hypothesis of an anticarcinogenic effect of neuroleptic medications is supported mainly by observations of reduced risks of several cancer types among schizophrenic patients (Dupont et al, 1986; Mortensen, 1989; Gulbinat et al, 1992; Mortensen, 1994). In this study, we investigated this hypothesis not only among schizophrenic patients but also among other patients receiving antipsychotic treatment. Our cohort study had the advantage of collecting information from population-based databases and registers with prospectively registered and virtually complete data on drug prescriptions, psychiatric and somatic hospital admissions and cancer diagnoses, thus minimising the possibility of selection and information bias. As neuroleptic medications are available only by prescription in Denmark, we are likely to have identified virtually all users of neuroleptic medications in the study population. The fact that a large proportion (37%) of the exposed group had been hospitalised with a psychiatric disorder might have led to some degree of misclassification in the analyses stratified by number of prescriptions, as these patients were probably treated with neuroleptic medication during their hospital admissions, information to which we had no access. Patients with chronic disorders such as psychiatric disorders are likely to be under closer medical supervision and diagnostic activity than the general population possibly resulting in surveillance bias. Conversely, under-ascertainment of cancer among those who are psychotic due to altered perception of physical symptoms and lower degree of adherence to diagnostic activities should also be considered. Although decreased surveillance cannot be ruled out, Danish autopsy studies do not support underdiagnosis of cancers in schizophrenic patients (Mortensen, 1987; Mortensen, 1994).
We observed a decreased risk for rectal cancer in both male and female users of neuroleptic medication. Although there was no clear trend by number of prescriptions or length of follow-up, risk was further reduced among long-term users with more than 10 prescriptions. Studies of patients with schizophrenia in Denmark and Finland have reported reduced risks for rectal cancer but not for colon cancer (Mortensen, 1989; Gulbinat et al, 1992; Mortensen, 1994; Lichtermann et al, 2001; Dalton et al, 2005) whereas we additionally observed a reduced incidence of colon cancer among female users of neuroleptic medication in the present study. Established risk factors for rectal and colon cancer are similar and we would expect exposure to mutual risk factors, such as high intake of alcohol, little exercise and high intake of meat or low intake of vegetables (Potter, 1999), to be more frequent among our cohort of neuroleptic users. Use of NSAIDs was frequent in the present cohort of neuroleptic users, although adjusting for this well established protective factor in colorectal cancer (Thun et al, 2002; Baron, 2003) did not alter the risk estimates associated with neuroleptic medication. In schizophrenia, the hypothesis of a lowered host susceptibility to cancer in general has been raised (Catts and Catts, 2000) but the present study indicated that the reduced risk for rectal cancer was not confined to patients with schizophrenia.
Prostate cancer was of a priori interest given the reduced risk reported in patients with schizophrenia (Mortensen, 1989; Mortensen, 1994; Lichtermann et al, 2001; Dalton et al, 2005) and the finding of a reduced risk among men with schizophrenia treated with high-dose phenothiazines (IRR, 0.33; 95% CI, 0.12–0.94) in a small Danish case–control study (Mortensen, 1992). Although in the present study there was no clear trend by number of prescriptions or length of follow-up we did find a 44% lower risk among long-term users with multiple prescriptions. Further, the reduced risk estimates were not confined to patients with schizophrenia but observed in all groups of users of neuroleptic medication, thus supporting an effect of the neuroleptic medication, rather than of schizophrenia.
A rationale for any association between use of neuroleptic medications and breast cancer is that conventional antipsychotic agents inhibit dopamine action at D2 receptors, leading to hyperprolactinemia (Halbreich et al, 2003). Epidemiological studies have produced inconsistent findings (Overall, 1978; Schyve, 1978; Wagner & Mantel, 1978; Kanhouwa et al, 1984; Nakane et al, 1986; Gulbinat et al, 1992; Halbreich et al, 1996; Lichtermann et al, 2001), although a more recent cohort study reported a relative risk of 1.16 (95% CI, 1.07–1.26) for breast cancer associated with use of neuroleptic medication, as well as a positive dose–response relationship (Wang et al, 2002). This study included information on a number of factors not included in previous studies, but residual confounding by factors such as reproductive history and family history of breast cancer or incomplete information on socioeconomic status and exogenous hormone use cannot be ruled out (Wang et al, 2002). In the present study, we were able to adjust for reproductive factors and use of HT and we found no evidence for an increased breast cancer risk.
Finally, our finding of an increased risk for lung cancer likely reflects the increased smoking among individuals with psychiatric disorders and in treatment with neuroleptic medication. We were able to include certain hospital diagnoses as proxies for heavy smoking and alcohol use; however, given the extent of evidence documenting the increased prevalence of smoking and alcohol use in long-term psychiatric patients (O'Farrell et al, 1983; Hughes et al, 1986; de Leon et al, 1995; Lasser et al, 2000), we believe that our results for lung cancer reflect residual confounding by smoking. In support of this, we also observed an increased risk for other smoking related cancers combined, that is cancer of the buccal cavity, oesophagus, pancreas, larynx, urinary bladder, and kidney (Dreyer et al, 1997) (adjusted IRR, 1.15; 95% CI, 0.97–1.37) among male users of neuroleptic medications (data not shown).
In conclusion, although no overall decrease in cancer incidence was found, the results of this study provide some support for an anticarcinogenic effect of neuroleptic use for cancers of the rectum, colon and prostate. Reassuringly, we found no increased risk for breast cancer.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH (1999) The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 46: 263–268
Baron JA (2003) Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res 37: 1–24
Catts VS, Catts SV (2000) Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res 41: 405–415
Dalton SO, Mellemkjær L, Thomassen L, Mortensen PB, Johansen C (2005) Risk for cancer in a cohort of patients hospitalized for schizophrenia in Denmark, 1969–1993. Schizophr Res 75: 315–324
de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM (1995) Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry 152: 453–455
Dreyer L, Winther JF, Pukkala E, Andersen A (1997) Tobacco smoking. APMIS 105: 9–47
Dupont A, Møller Jensen O, Strömgren E, Jablensky A (1986) Incidence of cancer in patients diagnosed as schizophrenic in Denmark. In ten Horn GHMM, Giel R, Gulbinat W, Henderson JH (eds) Psychiatric Case Registries in Public Health. Amsterdam: Elsevier Science Publisherws, pp 229–239
Gaist D, Sorensen HT, Hallas J (1997) The Danish prescription registries. Dan Med Bull 44: 445–448
Gulbinat W, Dupont A, Jablensky A, Jensen OM, Marsella A, Nakane Y, Sartorius N (1992) Cancer incidence of schizoprenic patients. Results of record linkage studies in three countries. Br J Psychiatr 161 (Suppl 18): 75–85
Hait WN, Lazo JS (1986) Calmodulin: a potential target for cancer chemotherapeutic agents. J Clin Oncol 4: 994–1012
Halbreich U, Kinon BJ, Gilmore JA, Kahn LS (2003) Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 28: Suppl 1: 53–67
Halbreich U, Shen J, Panaro V (1996) Are chronic psychiatric patients at increased risk for developing breast cancer? Am J Psychiatr 153: 559–560
Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA (1986) The prevalence of smoking among psychiatric outpatients. Am J Psychiatr 143: 993–997
Jones GRN (1985) Cancer therapy: phenothiazines in an unexpected role. Tumori 71: 563–569
Kanhouwa S, Gowdy JM, Solomon JD (1984) Phenothiazines and breast cancer. J Natl Med Ass 76: 785–788
Kelly JP, Rosenberg L, Palmer JR, Rao RS, Strom BL, Stolley PD, Zauber AG, Shapiro S (1999) Risk of breast cancer according to use of antidepressants, phenothiazines, and antihistamines. Am J Epidemiol 150: 861–868
Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH (2000) Smoking and mental illness. A population-based prevalence study. JAMA 284: 2606–2610
Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lönnqvist J (2001) Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatr 58: 573–578
Mortensen PB (1987) Cancerdiagnostik og autopsifrekvens blandt skziofrene patienter. Ugeskr Laeger 149: 1973–1975
Mortensen PB (1989) The incidence of cancer in schizophrenic patients. J Epidemiol Commun Health 43: 43–47
Mortensen PB (1992) Neuroleptic medication and reduced risk of prostate cancer in schizophrenic patients. Acta Psychiatr Scand 85: 390–393
Mortensen PB (1994) The occurence of cancer in first admitted schizophrenic patients. Schizophr Res 12: 185–194
Munk-Joergensen P, Mortensen PB (1997) The Danish Psychiatric Central Register. Dan Med Bull 44: 82–84
Nakane Y, Ohta Y (1986) The example of linkage with a cancer register. In ten Horn GHMM, Giel R, Gulbinat W, Henderson JH (eds) Psychiatric Case Registries in Public Health. Amsterdam: Elsevier, pp 240–245
Nielsen GL, Sorensen HT, Zhou W, Steffensen FH, Olsen J (1997) The pharmacoepidemiologic prescription database of North Jutland – a valid tool in pharmacoepidemiological research. Int J Risk Safety Med 10: 203–205
Nordenberg J, Fenig E, Landau M, Weizman R, Weizman A (1999) Effects of psychotropic drugs on cell proliferation and differentiation. Biochem Phamacol 58: 1229–1236
O'Farrell TJ, Connors GJ, Upper D (1983) Addictive behaviors among hospitalized psychiatric patients. Add Behav 8: 329–333
Olivarius NF, Hollnagel H, Krasnik A, Pedersen PA, Thorsen H (1997) The Danish National Health Service Register. A tool for primary health care research. Dan Med Bull 44: 449–453
Overall JE (1978) Prior psychiatric treatment and the development of breast cancer. Arch Gen Psychiatr 35: 898–899
Potter JD (1999) Colorectal cancer: molecules and populations. J Natl Cancer Inst 91: 916–932
Schyve PM, Smithline F, Meltzer HY (1978) Neuroleptic-induced prolactin level elevation and breast cancer. An emerging issue. Arch Gen Psychiatr 35: 1291–1301
Storm HH, Michelsen E, Clemmensen IH, Pihl J (1997) The Danish Cancer Registry: history, content, quality and use. Dan Med Bull 44: 535–539
Thun MJ, Henley SJ, Patrono C (2002) Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst 94: 252–266
Wagner S, Mantel N (1978) Breast cancer at a psychiatric hospital before and after the introduction of neuroleptic agents. Cancer Res 38: 2703–2708
Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, Avorn J (2002) Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatr 59: 1147–1154
Acknowledgements
The study was funded by the Danish Cancer Society and a grant from the International Epidemiology Institute. The study also obtained support from the Western Danish Research Forum for Health Sciences and Leo and Ingeborg Dannin Foundation for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Dalton, S., Johansen, C., Poulsen, A. et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br J Cancer 95, 934–939 (2006). https://doi.org/10.1038/sj.bjc.6603259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6603259
Keywords
This article is cited by
-
Association between cumulative exposure periods of flupentixol or any antipsychotics and risk of lung cancer
Communications Medicine (2023)
-
Inhibition of AMPK/PFKFB3 mediated glycolysis synergizes with penfluridol to suppress gallbladder cancer growth
Cell Communication and Signaling (2022)
-
Repurposed antipsychotic chlorpromazine inhibits colorectal cancer and pulmonary metastasis by inducing G2/M cell cycle arrest, apoptosis, and autophagy
Cancer Chemotherapy and Pharmacology (2022)
-
Post-diagnostic antipsychotic use and cancer mortality: a population based cohort study
BMC Cancer (2020)
-
The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis
Cell Death & Disease (2018)