Abstract
Although the PPARγ agonist troglitazone has been shown to induce growth inhibition of hepatocellular carcinoma (HCC) cells at high concentration, this study indicates troglitazone does not significantly inhibit the growth of HCC cells at clinically achievable concentrations (1–10 μ M), and this lack of activity could not be improved by the addition of 9-cis-retinoic acid. Furthermore, no synergistic effect was found between troglitazone and cytotoxic anticancer agents.
Similar content being viewed by others
Main
The peroxisome proliferator-activated receptor γ (PPARγ), a member of the nuclear hormone receptor superfamily, functions as a ligand-dependent transcription factor and plays an important role in several signalling pathways, including lipid metabolism, glucose homeostasis, and inflammation (Kersten et al, 2000). Heterodimerisation of PPARγ and retinoid X receptor (RXR) is required for binding to specific DNA response elements of target genes. Binding with either PPARγ or RXR ligands will elicit transcriptional activation of the target genes (Vanecq and Latruffe, 1999; Corton et al, 2000). Synergistic activation of downstream genes may occur when both ligands are present (Kliewer et al, 1992; Desvergene and Wahli, 1999). The thiazolidinediones were found to be specific agonists of PPARγ, with EC50 less than 1 μ M (Lehmann et al, 1995). The potency of the thiazolidinediones in activating PPARγ in vitro was found to correlate closely with their lipid- and glucose-lowering activity in vitro (Berger et al, 1996; Willson et al, 2001). Troglitazone, a thiazolidinedione derivative, has been demonstrated to induce adipocyte proliferation and differentiation with the concentrations of 0.5–5 μ M (Tafuri, 1996).
The anticancer activity of PPARγ agonists was first demonstrated in a liposarcoma model. Thiazolidinedione derivatives induced terminal differentiation of liposarcoma cells (Tontonoz et al, 1997; Demetri et al, 1999; Tsibris et al, 1999). Subsequent studies indicated that PPARγ agonists, at concentrations of 1–10 μ M, may induce growth inhibition in a variety of cancers, including cancers of breast, colon, and prostate (Kubota et al, 1998; Sarraf et al, 1998; Mehta et al, 2000). In addition to promotion of cell differentiation, the PPARγ agonists-induced growth inhibition may involve various mechanisms such as induction of cell cycle arrest, inhibition of DNA synthesis, and increase of cancer cell necrosis and apoptosis. Moreover, synergistic or additive effects of growth inhibition between PPARγ and RXRα agonists have been found in liposarcoma and breast cancer cells. Clinical trials exploring the feasibility of using PPARγ agonists in the treatment of human cancers are underway (Koeffler, 2003).
Recent studies have demonstrated that PPARγ agonists may induce growth inhibition in hepatocellular carcinoma (HCC) cells in a dose- and time-dependent manner (Koga et al, 2001; Rumi et al, 2001; Yoshizawa et al, 2002). Troglitazone, a thiazolidinedione derivative, enhanced the expression of the cyclin-dependent kinase inhibitors p21WAF1/Cip1 and p27Kip1 and resulted in cell cycle arrest at G0/G1 phase. PPARγ agonists may also augment Fas-mediated apoptosis of HCC cells induced by tumour necrosis factor α (Okano et al, 2002). However, these effects were observed at relatively high concentrations (20–50 μ M) of troglitazone, while the clinically achievable concentrations are around 2–5 μ M (Berger et al, 1996; Tafuri, 1996; Spencer and Markham, 1997; Willson et al, 2001). Therefore, the utility of PPARγ agonists for the treatment of HCC remained undetermined.
The current study was designed to address the following questions: (1) whether troglitazone alone, at clinically achievable concentrations, may be active against HCC cells; (2) whether troglitazone has a synergistic effect with RXRα agonists on growth inhibition of HCC cells; (3) whether the effects of troglitazone and RXRα agonists correlate with the expression of PPARγ and RXRα in HCC cells; and (4) whether troglitazone, at clinically achievable concentrations, may enhance the cytotoxic effects of major chemotherapeutic agents.
Materials and methods
Cell culture and reagents
A panel of HCC cell lines was tested in this study: Hep3B, HepG2, SNU-449 (Park et al, 1995, purchased from ATCC), PLC-5, Huh-7 (Nakabayashi et al, 1982), SK-hep1 (Fogh et al, 1977), HA-59T (Wuu et al, 1990) (gifts of Professor Ming-Yang Lai, Graduate Institute of Medicine, College of Medicine, National Taiwan University). MCF-7 (purchased from ATCC), a breast cancer cell line characterised by expression of RXRα and 9-cis retinoic acid (9-cis RA) growth inhibition, was used as positive control for RXRα expression and 9-cis-RA-induced cytotoxicity in this study (Gottardis et al, 1996). The HCC cells and MCF-7 were cultured in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, and maintained in a humidified incubator of 5% CO2 in air at 37°C.
Troglitazone was purchased from Cayman Chemical (Ann Arbor, MI, USA) and 9-cis-RA was purchased from Sigma (St Louis, MO, USA). We used 9-cis retinoic acid in our study because in earlier studies it has been demonstrated to have synergistic activity with PPARγ agonists in regulation of lipid metabolism and in a leiomyosarcoma model. Both troglitazone and 9-cis-RA were prepared in dimethyl suphoxide (DMSO) for cytotoxicity assay. The final concentration of DMSO was controlled at below 0.5%. The cytotoxic agents were provided by the following companies: gemcitabine, Eli Lilly; irinotecan, Rhone–Poulenc–Rorer; cisplatin, David Bull Laboratories; paclitaxel, Bristol-Myers-Squibb. Polyclonal mouse antibodies detecting PPARγ and rabbit antibodies detecting RXRα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal antibody detecting proliferating cell nuclear antigen (PCNA) was purchased from Chemicon (Temecula, CA, USA). The anti-PPARγ antibody we used in this study was mapped to the N-terminal of PPARγ (sc-7196, Santa Cruz, CA, USA). No cross reaction with other PPAR isoforms has been reported by the manufacturer. The dilution of antibodies was 1 : 1000 for primary antibodies and 1 : 2500 for secondary antibodies.
Extraction of nuclear proteins and Western blot analysis
The methods of nuclear protein extraction and Western blot analysis have been described previously (Chuang et al, 2002). Briefly, cells (3–5 × 106) were scraped from culture dishes, washed with iced phosphate-buffered saline, resuspended in 1 ml of buffer A (1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM PMSF, 1% Nonidet P-40, 10 mM HEPES, pH 7.9), and incubated for 10 min at 4°C. The cells were then centrifuged at 1500 rpm for 2 min at 4°C and the pellet was resuspended in 0.1 ml of buffer B (25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.2 mM PMSF, 20 mM HEPES, pH 7.9) and incubated for 20 min at 4°C. The cell suspension was then centrifuged at 14 000 rpm for 2 min at 4°C, and the supernatant was collected for protein quantification using a modified Lowry's method. The supernatant was stored in aliquots at −70°C. A measure of 30 μg of protein was deposited in each lane on the blot. The proteins were separated by SDS–PAGE and transferred to nitrocellulose membranes. The membranes were incubated with the appropriate primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies and a chemiluminescence agent (Santa Cruz). The proteins were then detected by roentgenography.
For each study at least three independent experiments, including independent cultures, protein collection and quantification, were done and separate blots were done to detect the expression of PPARγ and RXRα. The most representative figures were shown.
Cytotoxicity assay
Cytotoxicity was determined by a tetrazolium-based semiautomated colorimetric assay (MTT assay) (Mosmann, 1983). Three independent experiments of each cytotoxicity test have been done and the results were the mean of the three experiments. For each experiment at least three replicates were done. Cells were plated in 96-well plates 3–4 × 103 well−1) and incubated overnight. The drugs were added to the wells and the percentage of surviving cells was measured by MTT assay after continuous drug exposure for 72 h. Concurrent drug exposure was used to evaluate the combination effect of troglitazone with 9-cis-RA and troglitazone with four cytotoxic agents (gemcitabine, cisplatin, paclitaxel and irinotecan) that have different mechanisms of cytotoxicity. The IC50 of individual cell lines was calculated by using a linear regression based on the MTT assay results. The drug concentration corresponding to 50% inhibition of control was designated as IC50.
Results
Expression of PPARγ and RXRα and single-agent activity of troglitazone
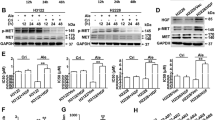
The nuclear expression of PPARγ and RXRα and the growth inhibitory effect of troglitazone on HCC cells are shown in Figure 1. All tested HCC cell lines expressed various levels of PPARγ and RXRα constitutively (Figure 1A). Troglitazone, up to 10 μ M, had no significant growth inhibitory activity on any of the HCC cell lines. Growth inhibition was noted at concentration more than 20 μ M, and the degree of inhibition did not correlate with the expression levels of PPARγ or RXRα (Figure 1B).
(A) Western blot analysis of PPARγ and RXRα expression in nuclear protein lysate of HCC cells. All tested HCC cell lines expressed various levels of PPARγ and RXRα constitutively. PCNA staining was used as loading control. (B) Growth inhibition of HCC cells induced by troglitazone determined by MTT assay. Growth inhibition was noted at concentration more than 20 μ M, and the degree of inhibition did not correlate with the expression levels of PPARγ or RXRα.
Combination activity of troglitazone and 9-cis RA
Addition of 10 μ M of 9-cis-RA caused 60% growth inhibition of MCF-7 cells but had no significant growth inhibitory effects on any of the HCC cells and did not affect the sensitivity of HCC cells to troglitazone (Figure 2).
Combination activity of troglitazone and anticancer agents
The IC50 of the four cytotoxic agents in three HCC cell lines, with and without the addition of 10 μ M of troglitazone, is listed in Table 1. The addition of troglitazone did not change the sensitivity of HCC cells toward any of the cytotoxic drugs.
Discussion
This study indicates that troglitazone does not significantly inhibit the growth of HCC cells at clinically achievable concentrations, and this lack of activity could not be improved by the addition of 9-cis-RA. Furthermore, no synergistic effect was found between troglitazone and four representative anticancer agents.
There was no apparent correlation between the degree of growth inhibition by troglitazone and the level of PPARγ or RXRα expression in our study. No correlation of the sensitivity of HCC cells to troglitazone with expression levels of PPARγ has been noted (Koga et al, 2001; Rumi et al, 2001; Okano et al, 2002). Similarly, previous studies for the sensitivity of cancer cells to retinoids have demonstrated that the growth inhibitory effects of retinoids did not correlate to the expression levels of different isoforms of retinoic acid receptors and RXRs (van der Leede et al, 1993). Other factors, including the levels of ‘free’ RXRα that is available for PPARγ-RXRα interaction, the content of other nuclear receptors, and the possible interaction among nuclear coactivators and corepressors, may play an important role in determining the sensitivity of cancer cells to the nuclear receptor agonists (Torchia et al, 1998).
Although troglitazone and other thiazolidinediones are considered specific PPARγ agonists, several lines of evidence suggest that the growth inhibition of HCC cells induced by high concentrations of troglitazone may occur through PPARγ-independent mechanisms. First, thiazolidinediones induce differentiation of adipose tissue and insulin sensitization at submicromolar levels and these effects are closely related to their binding affinity to PPARγ receptor (Lehmann et al, 1995; Goldstein, 2002). On the other hand, concentrations of troglitazone and other thiazolidinediones required to induce significant anticancer effect are usually 10 μ M or higher. Besides, no evident relationship between anticancer efficacy and the expression of PPARγ in cancer cells or the binding affinity of thiazolidinediones to PPARγ has been established. Second, addition of RXRα agonist may also improve the insulin-sensitising effect of thiazolidinediones by enhancing the formation of PPARγ–RXRα heterodimer (Mukherjee et al, 1997). This synergistic effect has also been found in liposarcoma and breast cancer cell lines but not in the present study, in spite of the constitutive expression of PPARγ and RXRα in the nuclei of HCC cells. Third, inhibition of cell growth by troglitazone through PPARγ-independent mechanisms has also been demonstrated in PPARγ−/− embryonal stem cells (Palakurthi et al, 2001). Troglitazone may inactivate the eukaryotic initiation factor 2 (eIF2) and abrogate the expression of G1 cyclins, thus resulting in cell cycle arrest at G1–S transition. Because the concentration of troglitazone needed to induce cell cycle arrest (10 μ M or higher) is significantly higher than the clinically achievable serum concentration (2–5 μ M), the clinical usefulness of troglitazone alone as an anticancer agent for HCC appears to be limited.
Troglitazone has also been demonstrated to induce cell cycle arrest through increased expression of the cyclin-dependent kinase inhibitors p21WAF1/Cip1, p27Kip1, and p18INK4c. Cell cycle modulation has been intensely investigated as a novel way to induce sensitisation to chemotherapeutic drugs as well as to inhibit cancer cell growth (Senderowicz and Sausville, 2000; Sherr, 2000). However, the concentration of troglitazone necessary to induce this effect is much higher than that achievable clinically. Four major anticancer agents were tested in this study for their effect in combination with troglitazone. The lack of synergistic activity between troglitazone and cytotoxic drugs in this study suggests that troglitazone may not be effective as a biochemical modulator for HCC.
The reason for the lack of efficacy of troglitazone as a biochemical modulator for HCC cells remained undetermined. Most of the HCC cell lines came from patients with surgically resected tumours. Some cell lines, such as PLC5, have been found to have integration of hepatitis B viral DNA into the host genome. The implication of viral DNA integration into host cells on drug sensitivity is not known, although in vitro data suggested that expression of hepatitis B viral proteins may increase apoptosis threshold and resistance to cytotoxic agents of cancer cells (Doong et al, 1998; Shih et al, 2000).
Nevertheless, the PPARγ agonists may have other anticancer effects. It has been demonstrated that PPARγ agonists are potent inhibitors of angiogenesis (Xin et al, 1999). Rosiglitazone, another thiazolidinedione derivative, has been shown to inhibit both primary tumour growth and metastasis (Panigrahy et al, 2002). The potential mechanisms of action include direct inhibition of endothelial cell proliferation, decrease of vascular endothelial growth factor production by tumour cells, and increased activity of the tissue inhibitor of matrix metalloproteinase (TIMP). Notably, the concentrations of rosiglitazone that had the strongest antiproliferative effect on endothelial cells (0.01–1 μ M) are clinically achievable. Therefore, it remains a possibility that new PPARγ agonists with novel antitumour mechanisms that are effective for the treatment of HCC can be developed.
In conclusion, troglitazone, at clinically achievable concentrations, does not appear to be active against HCC cells, either alone or in combination with 9-cis-RA or chemotherapeutic agents. Further exploration for new derivatives of PPARγ agonists with better antitumour activity in HCC is needed.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Berger J, Bailey P, Biswas C, Cullinan CA, Doebber TW, Hayes NS, Saperstein R, Smith RG, Leibowitz MD (1996) Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology 137: 4189–4195
Chuang SE, Yeh PY, Lu YS, Lai GM, Liao CM, Gao M, Cheng AL (2002) Basal levels and patterns of anticancer drug-induced activation of nuclear factor κB (NF-κB), and its attenuation by tamoxifen, dexamethasone, and curcumin in carcinoma cells. Biochem Pharmacol 63: 1709–1716
Corton JC, Anderson SP, Stauber A (2000) Central role of peroxisome proliferator-activated receptors in the action of peroxisome proliferators. Ann Rev Med 40: 491–518
Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S (1999) Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA 96: 3951–3956
Desvergene B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control and metabolism. Endocrinol Rev 20: 649–688
Doong SL, Lin MH, Tsai MM, Li TR, Chuang SE, Cheng AL (1998) Transactivation of the human MDR1 gene by hepatitis B virus X gene product. J Hepatol 29: 872–878
Fogh J, Fogh JM, Orfeo (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59: 221–225
Goldstein BJ (2002) Differentiating members of the thiazolidinedione class: a focus on efficacy. Diabetes Metab Res Rev 18: S16–S22
Gottardis MM, Lamph WW, Shalinsky DR, Wellstein A, Heyman RA (1996) The efficacy of 9-cis retinoic acid in experimental models of cancer. Breast Cancer Res Treat 38: 85–96
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405: 421–424
Kliewer A, Umesono K, Noonan DJ, Heyman RA, Evans RM (1992) Convergence of 9-cis retinoic acid and peroxisome proliferator signaling pathways through heterodimer of formation of their receptors. Nature 358: 771–774
Koeffler HP (2003) Peroxisome proliferator-activated receptor γ and cancers. Clin Cancer Res 9: 1–9
Koga H, Sakisaka S, Harada M, Takagi T, Hanada S, Taniguchi E, Okuyama T, Rukuda R, Nagasue N, Kinoshita Y (2001) Involvement of p21WAF1/Cip1, p27Kip1, and p18INK4c in troglitazone-induced cell-cycle arrest in human hepatoma cell lines. Hepatology 33: 1087–1097
Kubota T, Koshizuka K, Williamson EA, Asou H, Said JW, Holden S, Miyoshi I, Koeffler HP (1998) Ligand for peroxisome proliferator-activated receptor γ (troglitazone) has potent antitumor effect against human prostate cancer both in vitro and in vivo. Cancer Res 58: 3344–3352
Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ. J Biol Chem 270: 12953–12956
Mehta RG, Williamson E, Patel MK, Koeffler HP (2000) A ligand of peroxisome proliferators-activated receptor γ, retinoids, and prevention of preneoplastic mammary lesions. J Natl Cancer Inst 92: 418–423
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods 65: 55–63
Mukherjee R, Davies PJ, Crombie DL, Bischoff ED, Cesario RM, Jow L, Hamann LG, Boehm MF, Mondon CE, Nadzan AM, Paterniti JR, Heyman RA (1997) Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386: 407–410
Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J (1982) Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res 42: 3858–3863
Okano H, Shiraki K, Inoue H, Yamanaka T, Deguchi M, Sugimoto K, Sakai T, Ohmori S, Fujikawa K, Murata K, Nakano T (2002) Peroxisome proliferator-activated receptor γ augments tumor necrosis factor family-induced apoptosis in hepatocellular carcinoma. Anti-Cancer Drugs 13: 59–65
Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA (2001) Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor γ and mediated by inhibition of translation initiation. Cancer Res 61: 6213–6218
Panigrahy D, Singer S, Shen LQ, Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing S, Fletcher C, Fletcher JA, Hlatky L, Hahnfeldt P, Folkman J, Kaipainen A (2002) PPAR gamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. J Clin Invest 110: 923–932
Park JG, Lee JH, Kang MS, Park KJ, Jeon YM, Lee HJ, Kwon HS, Park HS, Yeo KS, Lee KU et al (1995) Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer 62: 276–282
Rumi MAK, Sato H, Ishihara S, Kawashima K, Hamamoto S, Kazumori H, Okuyama T, Rukuda R, Nagasue N, Kinoshita Y (2001) Peroxisome proliferator-activated receptor γ ligand-induced growth inhibition of human hepatocellular carcinoma. Br J Cancer 84: 1640–1647
Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Patridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM (1998) Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med 4: 1046–1052
Senderowicz AM, Sausville EA (2000) Preclinical and clinical development of cyclin-dependent kinase modulators. J Natl Cancer Inst 92: 376–387
Sherr CJ (2000) The Pezcoller lecture: cancer cell cycles revisited. Cancer Res 60: 3689–3695
Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL (2000) Hepatitis B virus X protein inhibits transforming growth factor-β-induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J Biol Chem 275: 25858–25864
Spencer CM, Markham A (1997) Troglitazone. Drugs 54: 89–101
Tafuri SR (1996) Troglitazone enhance differentiation, basal glucose uptake, and Glut1 protein levels in 3T3 adipocytes. Endocrinology 137: 4706–4712
Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Christopher DM, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, Evans RM, Spiegelman BM (1997) Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci USA 94: 237–241
Torchia J, Glass C, Rosenfeld MG (1998) Co-activators and co-repressors in the integration of transcriptional responses. Curr Opin Cell Biol 10: 373–383
Tsibris JCM, Porter KB, Jazayeri A, Tzimas G, Nau H, Huang H, Kuparadze K, Porter GW, O'Brien WF, Spellacy WN (1999) Human uterine leiomyomata express higher levels of peroxisome proliferator activated receptor γ, retinoid X receptor α, and all-trans retinoic acid than myometrium. Cancer Res 59: 5737–5744
van der Leede BM, van den Brink CE, van der Saag PT (1993) Retinoic acid receptor and retinoid X receptor expression in retinoic acid-resistant human tumor cell lines. Mol Carcinog 8: 112–122
Vanecq J, Latruffe N (1999) Medical significance of peroxisome proliferator-activated receptors. Lancet 354: 141–148
Willson TM, Lambert MH, Kliewer SA (2001) Peroxisome proliferators-activated γ and metabolic disease. Annu Rev Biochem 70: 341–367
Wuu KD, Wuu SW, Hu CP, Chang CM (1990) A human hepatocellular carcinoma cell line with multiple copies of structurally normal chromosomes. J Formos Med Assoc 89: 1–5
Xin X, Yang S, Kowalski J, Gerritsen ME (1999) Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem 274: 9116–9121
Yoshizawa K, Cioca DP, Kawa S, Tanaka E, Kiyosawa K (2002) Peroxisome proliferator-activated receptor γ ligand troglitazone induces cell cycle arrest and apoptosis of hepatocellular carcinoma cell lines. Cancer 95: 2243–2251
Acknowledgements
This study was supported by Grants NTUH-92-S076 from National Taiwan University Hospital and NHRI-CN-CA9201S(92A048), NHRI-CN-CA9201S(93A059) from National Health Research Institutes, Taiwan, ROC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shen, YC., Hsu, C., Chen, JY. et al. Lack of efficacy of troglitazone at clinically achievable concentrations, with or without 9-cis retinoic acid or cytotoxic agents, for hepatocellular carcinoma cell lines. Br J Cancer 91, 1561–1565 (2004). https://doi.org/10.1038/sj.bjc.6602200
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6602200
Keywords
This article is cited by
-
A novel chromonyl thiohydantoin with anti-proliferative action on primary hepatocellular carcinoma cells
Medicinal Chemistry Research (2018)