Abstract
This case–control study of 310 colorectal cancer cases and 1177 controls in a nested prospective, population-based cohort of Singapore Chinese subjects found a statistically significant association between the cyclooxygenase (COX)-2 −765G>C gene polymorphism and colon cancer risk among high consumers of dietary n-6 polyunsaturated fatty acids (odds ratio=2.38, 95% confidence interval=1.23–4.59).
Similar content being viewed by others
Main
Diet is believed to be the single most important contributor to colonic carcinogenesis (Tomatis et al, 1990). Experimental data have shown that saturated fatty acids (SFAs) and n-6 polyunsaturated fatty acids (PUFAs) have tumour-enhancing properties in the colon (Reddy and Maeura, 1984; Zhao et al, 1991, Woutersen et al, 1999). Epidemiological data suggest that increased consumption of all meat or red meat, which contains high levels of SFAs, is strongly associated with colorectal cancer (Giovannucci and Goldin, 1997; Sandhu et al, 2001), but there is only limited evidence on the role of dietary n-6 PUFAs (Zock and Katan, 1998; Flood et al, 2003).
The putative mechanism through which dietary n-6 PUFAs may enhance colonic carcinogenesis is the increased formation of prostaglandins, with the rate-limiting and committal step being mediated by the cyclooxygenase (COX)-2 enzyme (Dubois et al, 1998). Prostaglandins possess a wide spectrum of procarcinogenic properties (Handler et al, 1990; Cowlen and Eling, 1993; Coffey et al, 1997; Dermott et al, 1999). We therefore hypothesised that functional COX-2 gene polymorphisms may impact on the conversion of n-6 PUFAs into prostaglandins, with consequent change in level of cancer risk. A single nucleotide polymorphism (−765G>C) in the promoter region of the COX-2 gene was recently described (Papafili et al, 2002). We therefore investigated whether this COX-2 gene polymorphism was related to colorectal cancer risk within a population-based, prospective cohort of middle-aged and older Chinese men and women in Singapore.
Materials and methods
Study subjects
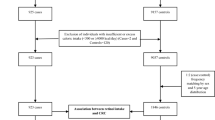
The study design and subject recruitment of the Singapore Chinese Health Study have been described (Hankin et al, 2001). Briefly, 63 257 Chinese women and men aged 45–74 years belonging to the Hokkien or Cantonese dialect group were enrolled in the study between April 1993 and December 1998. At recruitment, information on lifestyle factors and usual diet over the last year was obtained through in-person interviews. The dietary component of the questionnaire was validated through a series of 24-h food recalls (Hankin et al, 2001). Respondents were asked to choose from predefined frequency and portion size categories for each of the 165 listed food/beverage items that he/she consumed during the past 12 months. We used the Singapore Food Composition Table to estimate average daily intake of 96 nutrient and non-nutrient compounds for each study subject (Hankin et al, 2001). The Institutional Review Boards at the University of Southern California and the National University of Singapore had approved this study.
We identified incident colorectal cancer cases through the population-based cancer registry in Singapore (Chia et al, 2000). As of 30 April 2002, 592 colorectal cancer cases had occurred among cohort participants. All cases (including one carcinoid tumour and two in situ cancers) were histologically confirmed except three (ascertained by death records and clinical evidence). Details of the biospecimen collection, processing and storage procedures have been described (Koh et al, 2003). Briefly, we attempted to collect blood and single-void urine specimens from a random 3% sample of cohort enrollees. If the subject refused to donate blood, he/she was asked to donate buccal cells. We collected blood/buccal cell samples from 1194 subjects during April 1994–July 1999. Of these subjects, 13 developed colorectal cancer by 30 April 2002, and the remaining 1181 subjects constituted the referent group for the present study. We also attempted to collect blood/buccal cell and urine samples from all incident colorectal cancer cases. Of the 592 colorectal cancer cases, 312 (53%) donated blood/buccal cell samples.
COX-2 genotyping
Genomic DNA was extracted from buffy coats (228 cases and 895 controls) and buccal cell samples (84 cases and 286 controls) using a QIAamp 96 DNA Blood Kit (Qiagen, Valencia, CA, USA). A TaqMan assay for the −765G>C COX-2 polymorphism was developed using a TaqMan PCR Core Reagent kit (Applied Biosystems Inc., Foster City, CA, USA). The oligonucleotide primers for amplification of the polymorphic region of COX-2 were GC093 for (5′-CATTAACTATTTACAGGGTAACTGCTTAGG-3′) and GC093rev (5′-CCCCCTCCTTGTTTCTTGGA-3′). In addition, the fluorogenic oligonucleotide probes (TaqMan MGB Probes; ABI) used to detect each of the alleles were GC093F (5′-CTTTCCCGCCTCTCT-3′) labelled with 6-FAM to detect the G allele and GC093V (5′-CTTTCCCCCCTCTCT-3′) labelled with VIC to detect the C allele. Experimental samples were compared to 12 controls to identify the three genotypes at each locus (GG, GC, CC). All samples were processed without knowledge of their case/control status. Any samples that were outside the parameters defined by the controls were identified as noninformative and were retested. Four controls and two cases had noninformative COX-2 genotypes and were excluded from the present analysis.
Statistical analysis
Data were analysed by standard methods for unmatched case–control studies (Breslow and Day, 1980). Unconditional logistic regression models were used to examine the associations between COX-2 genotypes and risk of colorectal cancer, and their possible modification by n-6 PUFA intake. The associations were measured by odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) and P-values (two-sided). Limited by the very low frequency of the CC genotype (0.003), the GC and CC genotypes were combined when compared with the GG genotype. All ORs were adjusted for age (year) at recruitment, year of recruitment, gender, dialect group (Cantonese, Hokkien), level of education (no formal schooling, primary school, secondary school and higher), body mass index (<20, 20 to <24, 24 to <28, 28+ kg m−2), smoking status (never, exsmoker, current smoker), frequency of alcohol consumption (nondrinker, monthly drinker, weekly drinker, daily drinker), and familial history of colorectal cancer (yes, no).
Results
Of the 592 incident colorectal cancer cases, 282 were excluded from the present analysis due to unavailable blood/buccal cell samples (n=280) or noninformative COX-2 genotype (n=2). Cases included in the present study (n=310) were comparable to those excluded in terms of age (mean: 65.4 vs 66.1 years), but slightly different in gender (57 vs 49% male), dialect group (45 vs 37% Cantonese) and level of education (69 vs 60% attaining primary school education or higher).
In total, 180 (58%) cases had colon cancer, and the remaining cases had either rectal or rectosigmoid cancers. Table 1 shows the distributions of selected characteristics of colorectal cases and controls. Cases were older, less educated, more obese and more likely to smoke cigarettes than controls. Intakes of total calories, total fat, SFAs, monounsaturated fatty acids (MUFAs), PUFAs, n-3 PUFAs, n-6 PUFAs, fibre, calcium or folate were comparable between cases and controls.
Among control subjects, the G and C allele frequencies of the COX-2 genotype were 0.952 and 0.048, respectively, and the GG, GC and CC genotype frequencies were 0.907, 0.090 and 0.003, respectively. These genotypic distributions were in Hardy–Weinberg equilibrium (P=0.43). Overall, there was no association between colorectal cancer risk and COX-2 −765G>C genotype or n-6 PUFA intake (Table 2). When subjects were stratified into high (above median) vs low (below median) intake levels of n-6 PUFAs, a borderline statistically significant association between genotype and risk was observed among high consumers of n-6 PUFAs (OR=1.65, 95% CI=0.95–2.87), which was mainly confined to colon cancer (OR=2.38, 95% CI=1.23–4.59) (Table 3). There was no association between genotype and rectal cancer risk regardless of dietary n-6 PUFA intake levels. There was indication of an interaction effect between COX-2 genotype and dietary n-6 PUFAs in colon cancer (P=0.07), which was absent in rectal cancer (P=0.51). The corresponding P-value for the gene–diet interaction effect in colorectal cancer combined was 0.10.
Discussion
In this cohort of Singapore Chinese, we reported a statistically significant effect of the COX-2 −765G>C gene polymorphism on colon cancer risk among subjects with high intake of dietary n-6 PUFAs. Our data support the hypothesis that COX-2 exerts its effects on colon carcinogenesis through its influence on prostaglandin synthesis from n-6 PUFAs.
The current study has several strengths. (1) Our prospective study design precludes the possibility of recall bias. Furthermore, reliable dietary nutrient estimates including n-6 PUFAs were assessed using a validated food frequency questionnaire. (2) The nationwide cancer registry has been in place in Singapore since 1968 (Parkin et al, 2002), and migration out of Singapore has been negligible since inception of the study. This relatively complete ascertainment of cancer and death outcomes eliminates a concern for potential selection bias. (3) Study subjects originated from two contiguous regions in Southern China, resulting in a genetically homogeneous study population that facilitated the investigation of gene–disease associations. (4) Exposure information on other known/suspected risk factors for colorectal cancer was collected and accounted for in all analyses of gene–diet–cancer risk associations.
The chief limitation of our study is the lack of information on use of COX-2 inhibitors, which may bias the effect of COX-2 genotype on risk. However, if use of COX-2 inhibitors were to exert a confounding effect on the observed COX-2 genotype/colon cancer association, our inability to control for such confounding is likely to lead to an underestimation, rather than an overestimation, of the risk associated with the putative high-activity genotype. This is because use of COX-2 inhibitors is likely to be more common among subjects with more severe symptoms of inflammation, possibly due to the possession of the high activity COX-2 genotype. Another weakness of the present study is our relatively small number of cancer cases, which may result in less precise estimation of risk factor–disease associations.
The major n-6 PUFA in most diet is linoleic acid, the precursor of arachidonic acid. The latter is consequently converted to various prostaglandins, and COX is the crucial and rate-limiting enzyme for this conversion. There is compelling evidence that prostaglandins play important roles in colorectal carcinogenesis by enhancing cell proliferation and growth, promoting angiogenesis and inhibiting apoptosis (Cao and Prescott, 2002; Stoehlmacher and Lenz, 2003). COX-2 gene expression and its mRNA and protein levels were markedly elevated in most human colorectal cancers relative to adjacent normal mucosa (Kargman et al, 1995; Sano et al, 1995). It is hypothesised that the COX-2-associated effect on colorectal carcinogenesis is due to the increased production of prostaglandins from dietary n-6 PUFAs (Eberhart and Dubois, 1995). In support of this hypothesis, high dietary n-6 PUFAs has been shown to promote colon tumorigenesis by upregulating COX-2 expression in animal studies (Singh et al, 1997).
The human COX-2 gene is mapped to chromosome 1q25.2–q25.3 and spans about 8.3 kb pairs with 10 exons (Kosaka et al, 1994). Previous studies on the 5′ flanking region of the human COX-2 gene show that this region contains a canonical TATA box as well as several putative factor elements that are critical in inducing COX-2 gene transcription, such as Sp1, NF-κB, GRE (glucocorticoid) and IRE (insulin) elements (Tazawa et al, 1994; Yang et al, 1997). The region from nucleotide −827 to −454 has been described as a negative region since deletion of this region led to increased luciferase activity in reporter expression studies. The −765G>C mutation lies within this region, and is also within one of the five putative Sp1 elements (Yang et al, 1997). At present, data on the functionality of the −765G>C polymorphism and the direction/magnitude of change in protein expression/activity between the C and G alleles are limited and mixed (Papafili et al, 2002; Schneider et al, 2003).
In summary, the present study provides the first epidemiological evidence for a possible cause-and-effect connection between the production of prostaglandins from n-6 PUFAs through the enzymatic activity of COX-2, and increased risk of tumour development in the colon. Our novel findings require confirmation in larger studies with varying levels of substrate intake and genotype frequency.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Breslow NE, Day NE (1980) Statistical Methods in Cancer Research: Volume 1 – The Analysis of Case–Control Studies, IARC Scientific Publications No. 32. Lyon: IARC
Cao Y, Prescott SM (2002) Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol 190: 279–286
Chia KS, Seow A, Lee HP, Shanmugaratnam KS (2000) Cancer Incidence in Singapore, 1993–1997, Singapore Cancer Registry No. 5, Singapore
Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL, Morrow JD (1997) Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA 94: 657–662
Cowlen MS, Eling TE (1993) Effects of prostaglandins and hydroxyoctadecadienoic acid on epidermal growth factor-dependent DNA synthesis and c-myc proto-oncogene expression in Syrian hamster embryo cells. Biochim Biophys Acta 1174: 234–240
Dermott JM, Reddy MR, Onesime D, Reddy EP, Dhanasekaran N (1999) Oncogenic mutant of Gα12 stimulates cell proliferation through cycloxygenase-2 signaling pathway. Oncogene 18: 7185–7189
Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE (1998) Cyclooxygenase in biology and disease. FASEB J 12: 1063–1073
Eberhart CE, Dubois RN (1995) Eicosanoids and the gastrointestinal tract. Gastroenterology 109: 285–301
Flood A, Velie EM, Sinha R, Chaterjee N, Lacey Jr JV, Schairer C, Schatzkin A (2003) Meat, fat, and their subtypes as risk factors for colorectal cancer in a prospective cohort of women. Am J Epidemiol 158: 59–68
Giovannucci E, Goldin B (1997) The role of fat, fatty acids, and total energy intake in the etiology of human colon cancer. Am J Clin Nutr 66: 1564S–1571S
Handler JA, Danilowicz RM, Eling TE (1990) Mitogenic signaling by epidermal growth factor (EGF), but not platelet-derived growth factor, requires arachidonic acid metabolism in BALB/c 3T3 cells. Modulation of EGF-dependent c-myc expression by prostaglandins. J Biol Chem 265: 3669–3673
Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC (2001) Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 39: 187–195
Kargman SL, O’Neill GP, Vickers PJ, Evans JF, Mancini JA, Jothy S (1995) Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res 55: 2556–2559
Koh WP, Yuan JM, Sun CL, van den Berg D, Seow A, Lee HP, Yu MC (2003) Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res 63: 573–578
Kosaka T, Miyata A, Ihara H, Hara S, Sugimoto T, Takeda O, Takahashi E, Tanabe T (1994) Characterization of the human gene (PTGS2) encoding prostaglandin-endoperoxide synthase 2. Eur J Biochem 221: 889–897
Papafili A, Hill MR, Brull DJ, McAnulty RJ, Marshall RP, Humphries SE, Laurent GJ (2002) Common promoter variant in cyclooxygenase-2 represses gene expression: evidence of role in acute-phase inflammatory response. Arterioscler Thromb Vasc Biol 22: 1631–1636
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas D (eds) (2002) Cancer Incidence in Five Continents, IARC Scientific Publications No. 155. Lyon: IARC
Reddy BS, Maeura Y (1984) Tumor promotion by dietary fat in azoxymethane-induced colon carcinogenesis in female F344 rats: influence of amount and source of dietary fat. J Natl Cancer Inst 72: 745–750
Sandhu MS, White IR, McPherson K (2001) Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev 10: 439–446
Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T (1995) Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res 55: 3785–3789
Schneider S, Brabender J, Stoehlmacher J, Schneider PM, Uchida K, Yochim J, Yang D, Danenberg K, Lenz H-J, Danenberg PV (2003) Promoter variant in cyclooxygenase-2 is associated with lymph node involvement in patients with locally advanced non-small cell lung cancer. Proc Am Soc Clin Oncol 22: 652 (abstr 2621)
Singh J, Hamid R, Reddy BS (1997) Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of dietary fat during the postinitiation stage of colon carcinogenesis. Cancer Res 57: 3465–3470
Stoehlmacher J, Lenz HJ (2003) Cyclooxygenase-2 inhibitors in colorectal cancer. Semin Oncol 30: 10–16
Tazawa R, Xu XM, Wu KK, Wang LH (1994) Characterization of the genomic structure, chromosomal location and promoter of human prostaglandin H synthase-2 gene. Biochem Biophys Res Commun 203: 190–199
Tomatis L, Aitio A, Day NE, Heseltine E, Kaldor J, Miller AB, Parkin DM, Riboli E (eds) (1990) Cancer: Causes, Occurrence and Control, IARC Scientific Publications No. 100. Lyon: IARC
Woutersen RA, Appel MJ, van Garderen-Hoetmer A, Wijnands MVW (1999) Dietary fat and carcinogenesis. Mutat Res 443: 111–127
Yang X, Hou F, Taylor L, Polgar P (1997) Characterization of human cyclooxygenase 2 gene promoter localization of a TGF-β response element. Biochim Biophys Acta 1350: 287–292
Zhao LP, Kushi LH, Klein RD, Prentice RL (1991) Quantitative review of studies of dietary fat and rat colon carcinoma. Nutr Cancer 15: 169–177
Zock PL, Katan MB (1998) Linoleic acid intake and cancer risk: a review and meta-analysis. Am J Clin Nutr 68: 142–153
Acknowledgements
We thank Ms Siew-Hong Low of the National University of Singapore for supervising the field work of the Singapore Chinese Health Study, and Ms Kazuko Arakawa of the University of Southern California for the development and management of the cohort study database. The Singapore Chinese Health Study has been supported by Grants R01 CA55069, R35 CA53890 and R01 CA80205 from the National Cancer Institute, Bethesda, MD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Koh, WP., Yuan, JM., van den Berg, D. et al. Interaction between cyclooxygenase-2 gene polymorphism and dietary n-6 polyunsaturated fatty acids on colon cancer risk: The Singapore Chinese Health Study. Br J Cancer 90, 1760–1764 (2004). https://doi.org/10.1038/sj.bjc.6601797
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601797
Keywords
This article is cited by
-
Association of COX2 −765G>C promoter polymorphism and coronary artery disease in Korean population
Genes & Genomics (2019)
-
N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies
Cancer Causes & Control (2015)
-
Interactions between meat intake and genetic variation in relation to colorectal cancer
Genes & Nutrition (2015)
-
Functional promoter -765 G > C variant in COX-2 gene is associated with the susceptibility of breast cancer in Chinese Han women
Cancer Cell International (2014)
-
PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer
Genes & Nutrition (2013)