Abstract
Cyclo-oxygenase (COX)-2 is induced in various types of cancer tissues. Here, we demonstrate stromal expression of both COX-2 and microsomal prostaglandin E2 synthase (mPGES)-1 in gastrointestinal hamartomas developed in Lkb1+/−, Smad4+/− and Cdx2+/−mice. These results suggest that PGE2 produced by COX-2 and mPGES-1 plays an important role in hamartoma development regardless of the mutated genes causing hamartomas.
Similar content being viewed by others
Main
Using ApcΔ716 mouse mutant, a model for familial adenomatous polyposis (FAP), we demonstrated earlier that disruption of the gene encoding cyclo-oxygenase (COX)-2 or prostaglandin E2 (PGE2) receptor EP2 suppresses intestinal polyposis (Oshima et al, 1996; Sonoshita et al, 2001). These results indicate that PGE2 produced through the COX-2 pathway plays an important role in intestinal tumorigenesis. One of the PGE2 synthases, microsomal prostaglandin E2 synthase (mPGES)-1 appears to be responsible for PGE2 production in tumour tissues, because this enzyme is induced and functionally coupled with COX-2 in a human embryonic kidney cell line (Murakami et al, 2000). Likewise, COX-2 and mPGES-1 are induced simultaneously in human colorectal cancer tissues (Yoshimatsu et al, 2001), intestinal-type gastric adenocarcinomas (Van Rees et al, 2003) and ApcΔ716 mouse intestinal adenomas (Takeda et al, 2003).
Peutz–Jeghers syndrome (PJS) and juvenile polyposis syndrome (JPS) are autosomal dominant diseases characterised by hamartomatous polyps of the gastrointestinal tract with an increased risk of cancer development. Germ line mutations in the LKB1 (Hemminki et al, 1998; Jenne et al, 1998) and SMAD4 (Howe et al, 1998) are responsible for subpopulations of PJS and JPS, respectively. Gene-targeted mice heterozygous for Lkb1 and Smad4 develop gastrointestinal hamartomas that have histological characteristics similar to those of PJS and JPS, respectively (Takaku et al, 1999; Miyoshi et al, 2002). Recently, it has been reported that COX-2 expression is induced in hamartomatous polyps of PJS patients and Lkb1+/− mice (Rossi et al, 2002; de Leng et al, 2003; McGarrity et al, 2003), suggesting the roles of COX-2 in hamartoma development. However, COX-2 expression in other types of hamartomas has not yet been examined. Furthermore, it is important to determine whether the expression of mPGES-1 is also induced in hamartoma tissues as in intestinal adenomas and carcinomas.
Here we show that both COX-2 and mPGES-1 are induced in gastric hamartoma tissues of Lkb1+/− and Smad4+/− mice. In addition, we demonstrate induction of these enzymes also in Cdx2+/− mouse colonic hamartomas. These results strongly suggest that production of PGE2 is responsible for gastrointestinal hamartoma development as in intestinal adenomatous polyposis.
Materials and methods
All in vivo experiments were carried out with ethical committee approval and met the standards required by the UKCCCR guidelines (Workman et al, 1998). Constructions of Lkb1+/−, Smad4+/− and Cdx2+/− mutant mice have been described previously (Takaku et al, 1999; Tamai et al, 1999; Miyoshi et al, 2002). We used these mouse models to examine the expression patterns of COX-1, COX-2 and mPGES-1 in hamartomas that were caused by mutations in the putative genes, Lkb1, Smad4 and Cdx2, respectively. As the expression of COX-2 and mPGES-1 can be affected by various conditions such as infections, inflammations and host immune responses, it is important to use congenic mice bred in a specific pathogen-free (SPF) facility and compare them with the age-matched littermate controls. The results from these mouse experiments should provide important pieces of evidence applicable to human clinical research. Ages of mutant mice used in this study were 60–66, 76–90 and 20–35 weeks for Lkb1+/−, Smad4+/− and Cdx2+/−, respectively. Hamartomas were sampled from seven independent mice and used for further analysis.
For immunoblotting, tissue samples were homogenised and sonicated in lysis buffer (50 mM phosphate buffer pH 7.0, 100 mM NaCl, 2 mM EDTA) containing a protease inhibitor cocktail (Roche Diagnostics, Nonnenwald, Penzberg, Germany). After centrifugation at 10 000 × g at 4°C for 10 min, 40 μg of the supernatant protein was mixed with 6 × SDS sample buffer (350 mM Tris-HCl, pH 6.8, 36% glycerol, 10% SDS, 600 mM DTT), separated in SDS–polyacrylamide gels and transferred onto PVDF membranes. After blocking with 5% skimmed milk/Tris-buffered saline/Tween 20, membranes were incubated with an antibody for COX-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), COX-2, cPGES or mPGES-1 (Cayman Chemical, Ann Arbor, MI, USA) at 1000-fold dilution, or for β-actin (Sigma) at 5000-fold dilution. The ECL detection system (Amersham Pharmacia, Uppsala, Sweden) was used to detect the signals.
For immunohistochemistry, tissue samples were fixed in 4% paraformaldehyde, embedded in paraffin wax and sectioned at 4 μm. After pretreatment in 3% H2O2 in methanol, sections were boiled in 10 mM citrate buffer (pH 6.0) in a microwave oven for 5 min. Sections were blocked with 3% BSA-10% normal serum for 1 h and incubated with the primary antibody for COX-1, COX-2 or mPGES-1 at 400-fold dilution. Immunostaining signals were visualised using Vectastain Elite Kit (Vector Laboratories, Burlingame, CA, USA).
Results and discussion
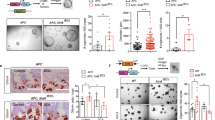
Hamartomatous polyp tissues were excised from the stomach of Lkb1+/− and Smad4+/− mice and from the colon of Cdx2+/− mice. We first examined the expression levels of COX enzymes and PGE2 synthases in these tumour tissues by immunoblotting (Figure 1). The expression of COX-2 and mPGES-1 was detected in all hamartomatous polyps, whereas these enzymes were rarely expressed in the normal tissues. On the other hand, COX-1 and cPGES were detected in both normal and hamartoma tissues. These results are consistent with our recent report that COX-1 and cPGES are expressed constitutively in the normal mouse intestines, whereas COX-2 and mPGES-1 are induced in the intestinal polyps of ApcΔ716 mice (Takeda et al, 2003).
We next determined by immunohistochemistry the localisation of COX-1, COX-2 and mPGES-1 in the hamartoma tissues of the Lkb1+/−, Smad4+/− and Cdx2+/− mice, respectively (Figure 2). In all samples, COX-1 was expressed in the stromal cells of hamartomas (Figure 2A, D, G) as well as in those of the normal mucosa (data not shown). Expression of COX-2 and mPGES-1 was detected in the stroma of hamartomas near the intestinal lumen, overlapping partly with the COX-1-expressing cells (Figure 2B–I). Moreover, cells expressing COX-2 and mPGES-1 appeared to be the same stromal cells showing a fibroblast-like morphology. There was no apparent difference in the expression patterns of COX-1, COX-2 and mPGES-1 among the hamartomas developing in different mutants. We did not find any difference in the expression levels and cell types among the individual mice of each model. These results indicate that COX-2 and mPGES-1 are induced in the stromal cells, and are consistent with our recent results with the intestinal adenomas in ApcΔ716 mice (Takeda et al, 2003). Namely, COX-2 and mPGES-1 are induced simultaneously in the COX-1-expressing stromal fibroblasts. Given that COX-2 and mPGES-1 are functionally coupled (Murakami et al, 2000), PGE2 levels in the hamartomas should be elevated significantly by simultaneous induction of both enzymes from the basal level that is secured by the COX-1 pathway alone. Although CDX2 mutations have not been detected in any hereditary hamartoma syndromes, it is conceivable that a subset of sporadic hamartomas contains CDX2 mutations. Regardless of the mutated genes that caused hamartomas, stromal PGE2 production appears to play a key role in the hamartoma expansion.
The PGE2 signalling stimulates tumour angiogenesis through the EP2 receptor (Seno et al, 2002), increases cell survival and motility (Sheng et al, 2001), inhibits host immune responses (Huang et al, 1998) and activates epidermal growth factor receptor (EGFR) (Pai et al, 2002). Accordingly, it is conceivable that stromal PGE2 in the hamartomas contributes to tumour expansion through these effects.
Inhibition of COX-2 by NSAIDs or COX-2-selective inhibitors suppresses intestinal polyposis in ApcΔ716 mice and FAP patients (Oshima et al, 1996; Steinbach et al, 2000). In addition, administration of COX-2 inhibitor to trefoil factor 1 (TFF1)-deficient mice suppresses gastric adenomas that are caused without Wnt signalling activation (Saukkonen et al, 2003). The results suggest that COX-2 induction in the tumour stroma is independent of the molecular mechanism that initiates tumorigenesis in the epithelial cells. These results, taken together, strongly suggest that COX-2 inhibitors, and possibly EP antagonists, are therapeutic agents effective for not only adenomatous polyposis but also hamartomas of the gastrointestinal tract. As hamartomatous polyps can progress into neoplastic tumours (Wang et al, 1999), COX-2 inhibitors may also turn out to be cancer chemopreventive agents suitable for hereditary hamartoma syndromes.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA (1998) A serine/threonine kinase gene defective in Peutz–Jeghers syndrome. Nature 391: 184–187
Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IPM, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA (1998) Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280: 1086–1088
Huang M, Stolina M, Sharma S, Mao JT, Zhu L, Miller PW, Wollman J, Herschman H, Dubinett SM (1998) Non-small cell lung cancer cyclooxygenase-2-dependent regulation of cytokine balance in lymphocytes and macrophages: up-regulation of interleukin 10 and down-regulation of interleukin 12 production. Cancer Res 58: 1208–1216
Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M (1998) Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18: 38–43
de Leng WWJ, Westerman AM, Westerman MAJ, de Rooij FWM, van Dekken H, de Goeij AFPM, Gruber SB, Wilson JHP, Offerhaus GJA, Giardiello FM, Keller JJ (2003) Cyclooxygenase 2 expression and molecular alterations in Peutz–Jeghers hamartomas and carcinomas. Clin Cancer Res 9: 3065–3072
McGarrity TJ, Peiffer LP, Amos CI, Frazier ML, Ward MG, Howett MK (2003) Overexpression of cyclooxygenase 2 in hamartomatous polyps of Peutz–Jeghers syndrome. Am J Gastroenterol 98: 671–678
Miyoshi H, Nakau M, Ishikawa T, Seldin FM, Oshima M, Taketo MM (2002) Gastrointestinal hamartomatous polyposis in Lkb1 heterozygous knockout mice. Cancer Res 62: 2261–2266
Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh-ishi S, Kudo I (2000) Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem 275: 32783–32792
Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, Trzaskos JM, Evans JF, Taketo MM (1996) Suppression of intestinal polyposis in ApcΔ716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 87: 803–809
Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS (2002) Prostaglandin E2 transactivates EGF receptor: A novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 8: 289–293
Rossi DJ, Ylikorkala A, Korsisaari N, Salovaara R, Luukko K, Launonen V, Henkemeyer M, Ristimaki A, Aaltonen LA, Makela TP (2002) Induction of cyclooxygenase-2 in a mouse model of Peutz–Jeghers polyposis. Proc Natl Acad Sci USA 99: 12327–12332
Saukkonen K, Tomasetto C, Narko K, Rio M-C, Ristimaki A (2003) Cyclooxygenase-2 expression and effect of celecoxib in gastric adenomas of trefoil factor 1-deficient mice. Cancer Res 63: 3032–3036
Seno H, Oshima M, Ishikawa T, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM (2002) Cyclooxygenase 2- and prostaglandin E2 receptor EP2-dependent angiogenesis in ApcΔ716 mouse intestinal polyps. Cancer Res 62: 506–511
Sheng H, Shao J, Washington MK, DuBois RN (2001) Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem 276: 18075–18081
Sonoshita M, Takaku K, Sasaki N, Sugimoto Y, Ushikubi F, Narumiya S, Oshima M, Taketo MM (2001) Acceleration of intestinal polyposis through prostaglandin receptor EP2 in ApcΔ716 knockout mice. Nat Med 7: 1048–1051
Steinbach G, Lynch PM, Phillips RKS, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B (2000) The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 342: 1946–1952
Takaku K, Miyoshi H, Matsunaga A, Oshima M, Sasaki N, Taketo MM (1999) Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res 59: 6113–6117
Takeda H, Sonoshita M, Oshima H, Sugihara K, Chulada PC, Langenbach R, Oshima M, Taketo MM (2003) Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Res 63: 4872–4877
Tamai Y, Nakajima R, Ishikawa T, Takaku K, Seldin MF, Taketo MM (1999) Colonic hamartoma development by anomalous duplication in Cdx2 knockout mice. Cancer Res 59: 2965–2970
Van Rees BP, Sivula A, Thoren S, Yokozaki H, Jakobsson PJ, Offerhaus GA, Ristimaki A (2003) Expression of microsomal prostaglandin E synthase-1 in intestinal type gastric adenocarcinoma and in gastric cancer cell lines. Int J Cancer 107: 551–556
Wang Z-J, Ellis I, Zauber P, Iwama T, Marchese C, Talbot I, Xue W-H, Yan Z-Y, Tomlinson I (1999) Allelic imbalance at the LKB1 (STK11) locus in tumors from patients with Peutz–Jeghers’ syndrome provides evidence for a hamartoma-(adenoma)-carcinoma sequence. J Pathol 188: 9–13
Workman P, Twentyman P, Balkwill F, Balman A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (second edition). Br J Cancer 77: 1–10
Yoshimatsu K, Golijanin D, Paty PB, Soslow RA, Jakobsson P-J, DeLellis RA, Subbaramaiah K, Dannenberg AJ (2001) Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res 7: 3971–3976
Acknowledgements
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Takeda, H., Miyoshi, H., Tamai, Y. et al. Simultaneous expression of COX-2 and mPGES-1 in mouse gastrointestinal hamartomas. Br J Cancer 90, 701–704 (2004). https://doi.org/10.1038/sj.bjc.6601584
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601584
Keywords
This article is cited by
-
Dysregulated CRTC1 activity is a novel component of PGE2 signaling that contributes to colon cancer growth
Oncogene (2016)
-
The inflammatory network in the gastrointestinal tumor microenvironment: lessons from mouse models
Journal of Gastroenterology (2012)
-
Molecular insights into Peutz-Jeghers syndrome: two probands with a germline mutation of LKB1
Journal of Gastroenterology (2008)