Abstract
Topotecan is currently approved for relapsed small-cell lung cancer and ovarian cancer. Topotecan's efficacy in the second-line setting and novel mechanism of action suggest broad antitumour activity. We utilised a clinically validated, cell-death, ex vivo assay in human tumour explants to examine the activity profile of topotecan alone and in combination with other antitumour agents. Serial dilutions of topotecan alone and in combination with other cytotoxic agents were applied to biopsy specimens of non-small-cell lung cancer (NSCLC) and breast, colon, and prostate cancers. Dose–response curves were interpolated to provide 50% lethal concentrations (LC50). The degree of synergy (by median effect) and normalised Z-scores (raw scores converted to relative activity distributed around the mean) were then computed. Single-agent activity was observed for topotecan in all four tumour types. In 57 chemotherapy-naive specimens, NSCLC revealed the highest activity, demonstrated by the lowest LC50 value (0.26±0.06 μg ml−1; P=0.002). Overall, previously treated and chemotherapy-naive specimens revealed no significant differences in mean LC50's. Synergy was observed for several combinations, including topotecan plus cisplatin in prostate and for topotecan plus 5-fluorouracil in breast cancers. The Z-score analyses conducted suggest activity for previously unexplored drug regimens, including topotecan plus 5-fluorouracil, vinorelbine, and mitomycin-C in NSCLC and breast cancer. Phase II studies are underway to determine the degree to which these ex vivo findings will translate into improved clinical results.
Similar content being viewed by others
Main
Topotecan, a semisynthetic, water-soluble derivative of camptothecin, is an inhibitor of topoisomerase I (Kingsbury et al, 1991). This derivative is more stable, has increased solubility, a shorter half-life, and decreased toxicity compared with its parent compound (Verweij et al, 1993; Burke and Mi, 1994; Rothenberg, 1997). Topoisomerase I is a nuclear enzyme that relieves torsional strain on supercoiled DNA and creates single-strand breaks during DNA replication. Topotecan prevents topoisomerase I from repairing the cleaved DNA, which results in double-stranded DNA breaks and eventually apoptosis. The unique mechanism of action of topotecan and lack of clinical crossresistance with other antineoplastic compounds suggest that topotecan has the potential for broad antitumour activity.
The activity of topotecan has been demonstrated in several open-label, randomised phase II and III trials. Currently, topotecan is approved for the treatment of relapsed small-cell lung cancer (SCLC) (Eckardt et al, 1996; Ardizzoni et al, 1997; Depierre et al, 1997; von Pawel et al, 1999, 2001) and ovarian cancer (Creemers et al, 1996; Kudelka et al, 1996; ten Bokkel Huinink et al, 1997; Bookman et al, 1998; Hoskins et al, 1998; McGuire et al, 2000). Although topotecan is typically given in a 30-min intravenous (i.v.) infusion on days 1–5 of a 21-day course, other clinical schedules have been investigated, including 21-day continuous i.v. infusion (Hochster et al, 1999), 3-day administration (Brown et al, 2000), single-day administration every 21 days (Wall et al, 1992), weekly bolus (Homesley et al, 2001), and weekly 24-h continuous i.v. infusion (Hoskins et al, 1998). In addition, an oral formulation of topotecan has also been investigated in SCLC (von Pawel et al, 2001) and ovarian cancer (Clarke-Pearson et al, 2001). Preliminary reports indicate that topotecan also has activity in non-small-cell lung cancer (NSCLC) (Lynch et al, 1994; Perez-Soler et al, 1996), cervical cancer (Fiorica et al, 2002), and haematologic malignancies (Beran et al, 1998). Additional studies are required to determine the activity of topotecan in other cancers.
Topotecan activity also suggests potential for synergy in combination with other cytotoxic agents. The predictable toxicity profile of topotecan makes it feasible to combine topotecan with other agents with nonoverlapping toxicities. Topotecan has been investigated in doublet and triplet combinations with several agents, including cisplatin, paclitaxel, and etoposide (Ardizzoni et al, 1999; Frasci et al, 1999; Hainsworth et al, 1999; Jacobs et al, 1999; O'Neill et al, 2001; Fiorica et al, 2002). Randomised trials are underway in several diseases to evaluate the efficacy of topotecan in combination with other agents.
Ex vivo analyses of human tumour primary cultures can provide disease-specific drug activity profiles and powerful insights useful in the development of phase I/II trials. The present study applied an ex vivo assay, based on the delayed loss of membrane integrity, to investigate the clinical potential of topotecan in human tumour biopsy specimens other than relapsed SCLC and ovarian cancer. Although earlier laboratory assays based on drug-induced growth inhibition (e.g. H3*-thymidine incorporation, clonogenic) have consistently failed to provide clinically relevant information, newer methods that incorporate cell-death measures as surrogates of drug-induced apoptosis have been shown to correlate with response, time to progression, and survival in human haematologic and solid tumours (Bosanquet et al, 1999; Cortazar and Johnson, 1999; Nagourney et al, 2000, 2003). The realisation that most chemotherapeutic agents exert their cytotoxic effects through apoptosis (Reed, 1999) supports the use of cell-death assay in the study of topotecan's antitumour activity in human neoplasms.
The principal focus of this study was the investigation of topotecan activity in biopsies of previously untreated NSCLC and breast, colorectal, and prostate carcinomas. We also assessed the synergy of topotecan in combination with other antineoplastic agents and compared the activity of topotecan in chemotherapy-naive vs previously treated specimens. Additional analyses compared the activity of topotecan with that of irinotecan, the related topoisomerase I inhibitor, in parallel studies.
Materials and methods
Tissue procurement and tumour-cell preparation
Patients undergoing surgical procedures for the management of NSCLC and breast, colorectal, or prostate cancers were offered the opportunity to participate in the study. All patients provided written informed consent allowing the release of tumour biopsy specimens for laboratory analysis. At the time of surgical exploration, tumour tissues removed from the patients were processed sterilely by the Department of Pathology at the Long Beach Memorial Medical Centre (Long Beach, CA, USA). After histologic diagnostic evaluations, the tissue samples were placed in sterile RPMI 1640 media containing 2 mM L-glutamine, 15% fetal calf serum, 100 IU ml−1 penicillin, and 100 μg ml−1 streptomycin (modified RPMI 1640), and were submitted directly to our laboratory. Samples obtained after hours were maintained at 4°C until processed (always <24 h). This study was approved by the Long Beach Memorial Medical Centre Human Subjects Committee.
Tumour specimens were mechanically disaggregated by sterile mincing with scalpels. Specimens were then incubated for 2 h in 0.8% collagenase IV and 0.002% DNaseI. Cells were isolated by density centrifugation over Ficoll–Hypaque, washed, and then resuspended in modified RPMI 1640. Cell suspensions were adjusted to 1 × 106 cells ml−1 and distributed into 96-well polypropylene culture plates (90 μl well−1). Serial dilutions of drugs and drug combinations in 10 μl volumes were added, and the plates were incubated at 37°C in 6% CO2 in a sterile, humidified environment for 72 h as described in the next section.
Drug exposure and measurement of cell viability
The dose-dependent cytotoxicity of drugs was investigated using a five-point dose–response curve. Serial dilutions of cytotoxic drugs were prepared in 0.15 M NaCl, and 10 μl of a drug solution was added to each well. Cells were incubated for 72 h with saline vehicle control (0.15 M NaCl) or in the presence of topotecan (range, 0.03–0.92 μg ml−1), alone or in combination at a fixed ratio in serial dilution with 5-fluorouracil (5-FU; range, 3.1–100 μg ml−1), cisplatin (range, 0.2–6.6 μg ml−1), doxorubicin (range, 0.04–1.2 μg ml−1), gemcitabine (range, 8.2–263 μg ml−1), mitomycin-C (range, 0.04–1.2 μg ml−1), mitoxantrone (range, 0.03–1.0 μg ml−1), nitrogen mustard (range, 0.17–5.4 μg ml−1), oxaliplatin (range, 0.3–10 μg ml−1), paclitaxel (range, 1.6–50 μg ml−1), or vinorelbine (range, 0.3–10 μg ml−1). After 72 h, 10 μl of phosphate-buffered saline containing 0.5% nigrosin-B and 1% Fast Green and 37 500 glutaraldehyde-fixed avian red blood cells (internal standard) were added to each well, and the samples were gently agitated to facilitate mixing. After 10 min, samples were aspirated and centrifuged onto a glass slide using a modified cytospin cassette. Air-dried samples were counterstained with modified haematoxylin and eosin. Tumour cell viability was determined as the ratio of living tumour cells over simultaneously counted avian red blood cells. Cell survival of drug-treated samples was expressed as a percentage of saline control values.
Calculation of LC50, synergy, and Z-score
The 50% lethal concentration (LC50) values were calculated using a least-squares line of best-fit generated from the five-point concentration curve, with the LC50 values interpolated from the curve. Synergy was determined using the median effect technique (Chou and Talalay, 1987, pp 37–64). In brief, dose–response curves and LC50 values were generated for topotecan both as a single agent and in combination with other agents. Comparison of the topotecan single-agent dose–response curves with the combination dose–response curves for a given tumour type allowed the determination of synergy, additivity, subadditivity, or antagonism. The term synergy only applied to tumour samples with 100% of the points on the combination dose–response curves falling above the line of additivity, while partial synergy applied to samples for which >50% of the points on the dose–response curve were above the line of additivity.
The overall mean LC50 (LC50T) and s.e.m. for all tumour types (s.e.m T) within our laboratory database were utilised to calculate Z-scores by the following formula:

The use of inferential statistics is predicated upon the central limit theorem (Feinstein, 1998), a fundamental sampling theorem that states that the distribution of a sample population is unimportant so long as the samples are drawn randomly in sufficient numbers from a parent population whose distribution need not have a normal (Gaussian) distribution (Hays, 1994).
By normalising the LC50 values for each single agent and/or combination around the mean LC50 values, samples found to be more resistant than average fell to the right of the mean, whereas those that were more sensitive than average fell to the left of the mean. This Z-score transformation is routinely used in the US by the National Cancer Institute for studies comparing microarray data and other analyses (Cheadle et al, 2003). It has been incorporated into the COMPARE statistical program (Shi et al, 1998), and into the latest version of the National Cancer Institute's public access MAExplorer bioinformatics tool (Lemkin et al, 2000).
Statistical methods
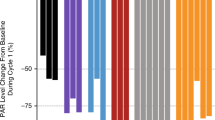
Statistical analysis of the study data was carried out using the analysis of variance program from the Statistical Package for the Social Sciences (Levine, 1991). The LC50 means for single-agent topotecan in untreated biopsies were compared across tumour types, with the criterion for statistical significance set at P⩽0.05. For comparisons of in vitro activity of topotecan in combination with other agents, LC50 values were normalised into Z-scores (with the mean set to 0 and variance from the mean in s.e.m. units of 1) (Kirk, 1982). This permitted direct comparisons of the sensitivity vs resistance of drug combinations across tumour types. Using these normalised LC50 values, graphic representations were generated depicting on the left side of the mean those samples more sensitive than average to drug combinations (negative range), and on the right side of the mean those more resistant than average to drug combinations (positive range). Finally, the activity of topotecan vs irinotecan in tumour specimens was assessed using a scatter gram graph and was statistically compared using the Pearson product-moment correlation from the same statistical package.
Results
Activity of single-agent topotecan
In aggregate, our topotecan database includes a total of 697 analyses, of which 380 are from NSCLC, and breast, colon, and prostate cancer biopsies that have been studies against single-agent topotecan. These include 149 specimens from chemotherapy-naive patients (untreated specimens) and 231 specimens from previously treated patients (previously treated specimens).
To assess the effect of prior chemotherapy exposure on the activity of topotecan, the LC50 values of topotecan in cells obtained from previously treated specimens were compared with those from untreated specimens (Figure 1). The mean LC50 value for all previously treated specimens was only marginally higher than that for the untreated specimens. However, when comparing previously treated LC50 values with untreated LC50 values within tumour types, several trends emerged. For instance, a comparison between the LC50 values of 135 previously treated breast cancer specimens with 33 untreated breast cancer specimens indicated a trend toward greater sensitivity in the previously treated specimens. The opposite trend was observed in colon cancer and NSCLC specimens. By rank order, previously untreated NSCLC specimens exhibited the lowest LC50 value (P=0.002), whereas previously untreated prostate cancer specimens had the highest LC50 value, suggesting that NSCLC and breast cancer might be better targets for topotecan than colon or prostate cancers.
LC50 values for single-agent topotecan in tumour biopsies taken from untreated and previously treated patients in the master database. Tumour biopsies from patients with breast cancer, colon cancer, NSCLC, or prostate cancer were incubated with various concentrations of topotecan, and the LC50 values were calculated. The numbers in parentheses indicate the number of samples for the indicated drug combination.
To address concerns that prior exposure to chemotherapy might pose a confounding variable, we procured 86 tumour specimens from previously untreated patients to conduct formal drug response and combination analyses. Viable tumour cells were successfully isolated from 74 (86%) of these specimens. Of 74 specimens, 57(77%), including 14 NSCLC and 18 breast, 13 prostate, and 12 colon cancer specimens, provided adequate cell yield for complete single-agent and a combination-agent analyses. Of the 74 specimens, 17(23%) provided adequate cell yield for partial analyses. The LC50 value (±s.e.m.) for single-agent topotecan in all 57 tumours was 0.43±0.04 μg ml−1. The LC50 values for each tumour type are shown in Table 1. The NSCLC samples demonstrated the lowest LC50 value at 0.26±0.06 μg ml−1 (P=0.002), suggesting that NSCLC is a potential target for topotecan treatment.
Synergy of topotecan combinations
The mechanism of action of topotecan suggests that combinations with other cytotoxic agents may provide increased antitumour activity. To assess this potential, a variety of topotecan–drug combinations were investigated for activity and degree of synergy. The LC50 values for topotecan in combination with other agents, at fixed drug ratios, and the degree of synergy for the different drug combinations by tumour type are provided in Table 2. It is worth noting that the degree of synergy varied by drug combination and tumour type.
Z-scores of topotecan combinations
Ex vivo drug concentrations were based on the clinically correlated database. Concentration ranges, by drug, varied by as much as three orders of magnitude. To compare antitumour activity in highly disparate concentration ranges directly, raw LC50 values were normalised using Z-scores, as summarised in Table 2 and illustrated in Figures 2 and 3. The data used in the generation of the s.e.m. values were obtained in previously untreated solid tumours in the laboratory database. These included NSCLC, colon, gastric, breast, ovarian, pancreatic, and uterine cancer specimens, ranging in number from 17 (mitoxantrone) to 53 (vinorelbine) with a median of 35 analyses per drug combination. A variety of combination regimens fell within the sensitive range of Z-scores for breast cancer and NSCLC tumour samples. The combination of doxorubicin and topotecan revealed significantly different Z-scores between breast and prostate cancer (P<0.001). Likewise, a comparison of prostate cancer, colon cancer, and NSCLC samples revealed significant differences in Z-scores across these tumour types for the combination of topotecan and oxaliplatin (P<0.01). Analyses of variance conducted across the four tumour types revealed significant differences in Z-scores for the topotecan/cisplatin combination (P<0.05). Finally, the topotecan/mitomycin-C combination resulted in significantly different Z-scores between NSCLC and colon cancer tumour samples (P<0.01). Only two combination regimens (topotecan/cisplatin and topotecan/nitrogen mustard) demonstrated Z-scores in the sensitive range in prostate cancer samples. None of the drug combinations tested on colon cancer samples had Z-scores within the sensitive range.
Z-scores of topotecan combination regimens in four tumour types. Z-scores of topotecan in biopsies from patients with breast cancer (open), colon cancer (dark grey), NSCLC (black), and prostate cancer (light grey). The numbers in parentheses indicate the number of samples for the indicated drug combination. NSCLC=non-small-cell lung cancer; 5-FU=5-fluorouracil; CDDP=cisplatin; DOX=doxorubicin; GEM=gemcitabine; MITX=mitoxantrone; NAV=vinorelbine; NM=nitrogen mustard; TAX=paclitaxel; L-OHP=oxaliplatin; MMC=mitomycin-C.
Relative in vitro activity of topotecan vs irinotecan. A scatterplot of the 50% lethal concentration values for topotecan and irinotecan in 239 tumour biopsy samples. A correlation coefficient of 0.54 indicates significant correlation between the antitumour activity of topotecan and irinotecan in vitro (P<0.0005).
Topotecan vs irinotecan
A unique advantage of these ex vivo analyses is their ability to compare drugs in parallel studies conducted on individual patient tumour specimens. Irinotecan, another topoisomerase I inhibitor, is a prodrug activated by carboxylesterase to form the active metabolite SN38. While spontaneous degradation or activation by carboxylesterases present in culture media is known to occur, and provides cytotoxic activity in vitro, some variability between irinotecan and topotecan would be expected based on topotecan's direct cytotoxic actions. When we directly compared the activity of topotecan against that of irinotecan in 239 chemonaive and previously treated human tumour specimens, there was a significant correlation between the LC50 values for topotecan vs irinotecan (P<0.0005). At the time of initial calculation, a portion of the LC50 values were rounded to the nearest 0.1 μg ml−1 value.
Discussion
The clinical efficacy and favourable safety profile of topotecan in the treatment of relapsed SCLC and ovarian cancer patients, and topotecan's novel mechanism of action have prompted investigations into its use in other solid tumours. Ex vivo analyses, based on clinically validated end points, can identify disease-specific drug activity profiles and offer a rational approach to drug development. We applied ex vivo analyses to evaluate the activity of single-agent topotecan and topotecan combinations in human NSCLC and breast, colon, and prostate cancer specimens.
Ex vivo topotecan activity was highest for NSCLC and breast cancer specimens and lowest for prostate and colon cancer specimens. This suggests that NSCLC may be more sensitive to single-agent topotecan than prostate cancers.
Several phase II trials have investigated the use of topotecan in the treatment of advanced NSCLC. Perez-Soler et al (1996) and Weitz et al (2000) reported overall response rates of 15 and 16%, respectively, in patients treated with topotecan, and Lynch et al (1994) reported stable disease in 55%. In contrast, and consistent with the results of the current study, single-agent topotecan has shown less promise in patients with metastatic, hormone-refractory prostate cancer (Hudes et al, 1995). The synergy results for prostate and other cancers may nonetheless offer fertile avenues for future investigations with topotecan. A possible explanation for the difference between in vitro and in vivo activity of single-agent topotecan in NSCLC is a pharmacokinetic effect. The inherent activity (reflected ex vivo) for topoisomerase I inhibitors may not be optimally exploited using current administration schedules. Recent observations with alternative schedules – including oral, long-term i.v., or the weekly administration of topotecan – suggest that these regimens may prove superior to the current 5-day topotecan schedules in those diseases that are most inherently sensitive to topotecan, with NSCLC appearing to be an attractive target.
In our study, several topotecan combinations demonstrated synergy. Among the most active combinations was that of topotecan plus 5-FU in breast cancer, which demonstrated synergy in 57% of specimens. It must be noted that the degree of synergy (combinatorial advantage) is independent of the relative antitumour activity (i.e. LC50). However, synergy, defined as pharmacologic supraadditivity, may occur at LC50 values that have no clinical relevance. Therefore, it is advantageous to examine the degree of synergy in the context of the relative activity, and Z-scores facilitate this comparison. The Z-score for topotecan/5-FU in breast cancer fell in the sensitive range. Based on both synergy and sensitivity (by Z-score) findings for this combination in breast cancer patients, a phase II clinical trial has been initiated (phase II study of topotecan plus capecitabine for recurrent, locally advanced or metastatic breast cancer). This study will examine the clinical activity of this combination and correlate clinical response with ex vivo results for each patient.
To date, breast cancer has not been a target for topotecan therapy. Levine et al (1999) reported a response rate of 10% in a study of topotecan in relapsed breast cancer conducted by the Cancer and Leukaemia Group B. However, Japanese investigators reported a 23% response rate for the closely related irinotecan in patients with advanced breast cancer (Taguchi et al, 1994). The irinotecan schedules (based on weekly dosing) compared with the topotecan schedules (based on consecutive 5-day dosing) may, in part, explain the differences in overall response rates. By modifying the topotecan schedule to days 1 and 8, we more closely approximate the irinotecan administration schedule in our current study. The concordance between irinotecan and topotecan observed in our parallel analyses suggest that future ex vivo analyses could compare topotecan with other topoisomerase I inhibitors (e.g. 9-aminocamptothecin or 9-nitrocamptothecin).
Several topotecan combinations demonstrated synergy in NSCLC specimens, including topotecan/mitomycin-C (38%) and topotecan/cisplatin (31%). Both combinations revealed favourable Z-scores. Preliminary results for topotecan/cisplatin in NSCLC patients have provided overall response rates ranging from 14 to 31% (Raymond et al, 1997; Wiesenfeld et al, 1997). The high incidence of haematologic and nonhaematologic toxicity limited the use of this combination. Alternate dosing schedules for topotecan/cisplatin, or the substitution of carboplatin for cisplatin, might prove more tolerable. A phase II study of topotecan/carboplatin in NSCLC patients provided a clinical benefit in 49% of patients and a 1-year survival rate of 32% (Pujol et al, 2001). No episodes of nephrotoxicity, ototoxicity, or neurotoxicity were reported. Although synergy was demonstrated in <20% of specimens in NSCLC for topotecan/vinorelbine, the Z-scores for this combination, and those for topotecan/gemcitabine, fell within the sensitive range. Vinorelbine provides single-agent activity with overall response rates ranging from 12 to 20% in NSCLC (Crawford et al, 1996; The Elderly Lung Cancer Vinorelbine Italian Study Group, 1999). The ex vivo results revealing activity for topotecan/vinorelbine in NSCLC are consistent with the report of Stupp et al (2001), which provided a response rate of 42% in NSCLC patients treated with this combination
Ex vivo activity profiles also provide the opportunity to develop novel triplet regimens. The favourable activity observed for the doublets of topotecan plus cisplatin and topotecan plus gemcitabine paralleled our ex vivo results for the three-drug combination in NSCLC specimens (unpublished observations). Guarino et al (2002) reported a 38% objective response rate and 1-year survival of 33% in patients with NSCLC who received this weekly regimen. Toxicity was extremely mild with only one of 30 patients experiencing a grade IV adverse event (leukopenia). A second phase II trial is under final development to further evaluate this triplet in NSCLC (phase II study of hycamtin, cisplatinum, and gemcitabine in advanced NSCLC).
Several other observations were of interest. Despite the low single-agent activity of topotecan in prostate cancer specimens, the favourable degrees of synergy and activity for topotecan/cisplatin in this disease could provide insights for future investigation. On the contrary, the high degree of synergy for topotecan/mitomycin-C in colon cancer specimens when combined with the resistant Z-score for this combination in colon specimens argues against clinical evaluation. Although single-agent topotecan is widely used for the treatment of relapsed ovarian cancer, combinations identified in this study may offer additional insights. The high degree of synergy that we reported previously for topotecan/gemcitabine in ovarian cancer specimens (Su et al, 2001) is supported by the results of a phase II study, in which seven of 11 (64%) patients with relapsed ovarian cancer achieved objective responses with this combination (Sehouli et al, 2001). Topotecan combinations might also be investigated in diseases that are historically resistant to chemotherapy. Fiorica et al (2002) reported a 28% (nine of 32) overall response rate for topotecan/cisplatin in patients with recurrent cervical cancer.
In summary, topotecan reveals activity in human NSCLC and in breast, prostate, and colon cancer primary cultures. Previously, unrecognised synergy between topotecan and other agents have allowed the development of novel phase II clinical trials in NSCLC and breast cancers. Trials are also in development to evaluate topotecan in cervical, colorectal, prostate, and haematologic malignancies.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ardizzoni A, Hansen H, Dombernowsky P, Gamucci T, Kaplan S, Postmus P, Giaccone G, Schaefer B, Wanders J, Verweij J (1997) Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. J Clin Oncol 15: 2090–2096
Ardizzoni A, Manegold C, Gaafar R, Buchholdz E, Debruyne C, Damen S, Curran D, King K, Giaccone G (1999) Combination chemotherapy with cisplatin and topotecan as second-line treatment of sensitive (S) and refractory (R) small cell lung cancer (SCLC): an EORTC LCCG phase II study (abstract). Proc Am Soc Clin Oncol 18: 471a
Beran M, Estey E, O'Brien SM, Giles FJ, Koller CA, Kornblau S, Keating M, Kantarjian HM (1998) Results of topotecan single-agent therapy in patients with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leukemia Lymphoma 31: 521–531
Bookman MA, Malmström H, Bolis G, Gordon A, Lissoni A, Krebs JB, Fields SZ (1998) Topotecan for the treatment of advanced epithelial ovarian cancer: an open-label phase II study in patients treated after prior chemotherapy that contained cisplatin or carboplatin and paclitaxel. J Clin Oncol 16: 3345–3352
Bosanquet AG, Johnson SA, Richards SM (1999) Prognosis for fludarabine therapy of chronic lymphocytic leukaemia based on ex vivo drug response by DISC assay. Br J Haematol 106: 71–77
Brown III JV, Peters III WA, Rettenmaier MA, Karlan BY, Dillman RA, Smith MR, Drescher CW, Micha JP (2000) A phase I trial of a 3-day topotecan q 21 days for recurrent epithelial cancers of the ovary, fallopian tube, and peritoneum. Gynecol Oncol 79: 495–498
Burke TG, Mi Z (1994) The structural basis of camptothecin interactions with human serum albumin: impact on drug stability. J Med Chem 37: 40–46
Cheadle C, Vawter MP, Freed WJ, Becker KG (2003) Analysis of microarray data using Z score transformation. J Mol Diagn 5: 73–81
Chou T-C, Talalay P (1987) Applications of the median-effect principle for the assessment of low-dose risk of carcinogens and for the quantitation of synergism and antagonism of chemotherapeutic agents. In New Avenues in Developmental Cancer Chemotherapy Harrap KR, Connors TA (eds) pp 37–64. Orlando, FL: Academic Press Inc.
Clarke-Pearson DL, Van Le L, Iveson T, Whitney CW, Hanjani P, Kristensen G, Malfetano JH, Beckman RA, Ross GA, Lane SR, DeWitte MH, Fields SZ (2001) Oral topotecan as single-agent second-line chemotherapy in patients with advanced ovarian cancer. J Clin Oncol 19: 3967–3975
Cortazar P, Johnson BE (1999) Review of the efficacy of individualized chemotherapy selected by in vitro drug sensitivity testing for patients with cancer. J Clin Oncol 17: 1625–1631
Crawford J, O'Rourke M, Schiller JH, Spiridonidis CH, Yanovich S, Ozer H, Langleben A, Hutchins L, Koletsky A, Clamon G, Burman S, White R, Hohneker J, Spiridonidis CH (1996) Randomized trial of vinorelbine compared with fluorouracil plus leucovorin in patients with stage IV non-small-cell lung cancer. J Clin Oncol 14: 2774–2784
Creemers GJ, Bolis G, Gore M, Scarfone G, Lacave AJ, Guastalla JP, Despax R, Favalli G, Kreinberg R, Van Belle S, Hudson I, Verweij J, ten Bokkel Huinink WW (1996) Topotecan, an active drug in the second-line treatment of epithelial ovarian cancer: results of a large European phase II study. J Clin Oncol 14: 3056–3061
Depierre A, von Pawel J, Hans K, Moro D, Clark P, Gatzemeier U, Paillot N, Scheithauer W, Carmichael J, Santoro A, Ross G, Marangolo M (1997) Evaluation of topotecan (Hycamtin™) in relapsed small cell lung cancer (SCLC). A multicentre phase II study (abstract). Lung Cancer 18(Suppl. 1): 35
Eckardt J, Gralla R, Palmer MC, Gandara D, Laplante J, Sandler A, Fields SZ, Fitts D, Broom C (1996) Topotecan (T) as second-line therapy in patients (Pts) with small cell lung cancer (SCLC): a phase II study (abstract). Ann Oncol 7(Suppl. 5): 107
Feinstein AR (1998) Clinical Epidemiology: The Architecture of Clinical Research. Philadelphia: W.B. Sanders
Fiorica J, Holloway R, Ndubisi B, Orr J, Grendys E, Boothby R, DeCesare S, LaPolla J, Hoffman M, Patel J (2002) Phase II trial of topotecan and cisplatin in persistent or recurrent squamous and nonsquamous carcinomas of the cervix. Gynecol Oncol 85: 89–94
Frasci G, Panza N, Comella P, Carteni G, Guida T, Nicolella GP, Natale M, Lombardi R, Apicella A, Pacilio C, Gravina A, Lapenta L, Comella G (1999) Cisplatin–topotecan–paclitaxel weekly administration with G-CSF support for ovarian and small-cell lung cancer patients: a dose-finding study. Ann Oncol 10: 355–358
Guarino MJ, Schneider CJ, Grubbs SS, Biggs DD, Himelstein AL, Hogaboom K, Tilak S (2002) A dose-escalation study of weekly topotecan, cisplatin, and gemcitabine front-line therapy in patients with inoperable non-small cell lung cancer. Oncologist 7: 509–515
Hainsworth JD, Burris III HA, Morrissey LH, Greco FA (1999) Phase I trial of paclitaxel, carboplatin, and topotecan with or without filgrastim (granulocyte-colony stimulating factor) in the treatment of patients with advanced, refractory cancer. Cancer 85: 1179–1185
Hays WL (1994) Statistics 5th edn. International Thomson Publishing.
Hochster H, Wadler S, Runowicz C, Liebes L, Cohen H, Wallach R, Sorich J, Taubes B, Speyer J (1999) Activity and pharmacodynamics of 21-day topotecan infusion in patients with ovarian cancer previously treated with platinum-based chemotherapy. J Clin Oncol 17: 2553–2561
Homesley HD, Hall DJ, Martin DA, Lewandowski GS, Vaccarello L, Nahhas WA, Suggs CL, Penley RG (2001) A dose-escalating study of weekly bolus topotecan in previously treated ovarian cancer patients. Gynecol Oncol 83: 394–399
Hoskins P, Eisenhauer E, Beare S, Roy M, Drouin P, Stuart G, Bryson P, Grimshaw R, Capstick V, Zee B (1998) Randomized phase II study of two schedules of topotecan in previously treated patients with ovarian cancer: a National Cancer Institute of Canada Clinical Trials Group study. J Clin Oncol 16: 2233–2237
Hudes GR, Kosierowski R, Greenberg R, Ramsey HE, Fox SC, Ozols RF, McAleer CA, Giantonio BJ (1995) Phase II study of topotecan in metastatic hormone-refractory prostate cancer. Invest New Drugs 13: 235–240
Jacobs SA, Jett JR, Belani CP, Long GS, Day RD, Kim HD, Levitt ML, Woolley PV (1999) Topotecan and paclitaxel, an active couplet, in untreated extensive disease small cell lung cancer (abstract). Proc Am Soc Clin Oncol 18: 470a
Kingsbury WD, Boehm JC, Jakas DR, Holden KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF, Johnson RK, Hertzberg RP (1991) Synthesis of water-soluble (aminoalkyl) camptothecin analogues: inhibition of topoisomerase I and antitumor activity. J Med Chem 34: 98–107
Kirk RE (1982) Experimental Design: Procedure for The Behavorial Sciences. Pacific Grove, CA: Brooks/Cole Publishing Company
Kudelka AP, Tresukosol D, Edwards CL, Freedman RS, Levenback C, Chantarawiroj P, Gonzalez de Leon C, Kim EE, Madden T, Wallin B, Hord M, Verschraegen C, Raber M, Kavanagh JJ (1996) Phase II study of intravenous topotecan as a 5-day infusion for refractory epithelial ovarian carcinoma. J Clin Oncol 14: 1552–1557
Lemkin PF, Thornwall CG, Walton KD, Hennighausen L. (2000) Themicroarray explorer to for data mining of cDNA microarrays: application for the mammary gland. Nucleic Acids Res 28: 4452–4459
Levine G (1991) A Guide to SPSS for Analysis of Variance. Hove: Lawrence Erlbaum Associates
Levine EG, Cirrincione CT, Szatrowski TP, Canellos G, Norton L, Henderson IC (1999) Phase II trial of topotecan in advanced breast cancer: a Cancer and Leukemia Group B study. Am J Clin Oncol 22: 218–222
Lynch Jr TJ, Kalish L, Strauss G, Elias A, Skarin A, Shulman LN, Posner M, Frei III E (1994) Phase II study of topotecan in metastatic non-small-cell lung cancer. J Clin Oncol 12: 347–352
McGuire WP, Blessing JA, Bookman MA, Lentz SS, Dunton CJ (2000) Topotecan has substantial antitumor activity as first-line salvage therapy in platinum-sensitive epithelial ovarian carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol 18: 1062–1067
Nagourney R, Brewer C, Radecki S, Kidder W, Sommers B, Evans S, Minor D, DiSaia PJ (2003) A phase II trial of gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed ovarian cancer patients. Gynecol Oncol 88: 35–39
Nagourney RA, Link JS, Blitzer JB, Forsthoff C, Evans SS (2000) Gemcitabine plus cisplatin repeating doublet therapy in previously treated, relapsed breast cancer patients. J Clin Oncol 18: 2245–2249
O'Neill P, Clark PI, Smith D, Marshall E, Hannigan K, Ross G (2001) A phase I trial of a 5-day schedule of intravenous topotecan and etoposide in previously untreated patients with small-cell lung cancer. Oncology 61(Suppl. 1): 25–29
Perez-Soler R, Fossella FV, Glisson BS, Lee JS, Murphy WK, Shin DM, Kemp BL, Lee JJ, Kane J, Robinson RA, Lippman SM, Kurie JM, Huber MH, Raber MN, Hong WK (1996) Phase II study of topotecan in patients with advanced non-small-cell lung cancer previously untreated with chemotherapy. J Clin Oncol 14: 503–513
Pujol JL, von Pawel J, Tumolo S, Martoni A, Hearn S, Fields SZ, Ross G (2001) Preliminary results of combined therapy with topotecan and carboplatin in advanced non-small-cell lung cancer. Oncology 61(Suppl. 1): 47–54
Raymond E, Burris HA, Rowinsky EK, Eckardt JR, Rodriguez G, Smith L, Weiss G, Von Hoff DD (1997) Phase I study of daily times five topotecan and single injection of cisplatin in patients with previously untreated non-small-cell lung carcinoma. Ann Oncol 8: 1003–1008
Reed JC (1999) Dysregulation of apoptosis in cancer. J Clin Oncol 17: 2941–2953
Rothenberg ML (1997) Topoisomerase I inhibitors: review and update. Ann Oncol 8: 837–855
Sehouli J, Lichtenegger W, Hindenburg HJ, Klare P, Keil E, Heinrich G, Elling D, Hieronimus-Reichel A, Oskay G, Blohmer J, Camara O, Ledwon P, Stengel D (2001) A phase II study of topotecan plus gemcitabine in the treatment of patients with relapsed ovarian cancer after failure of first-line chemotherapy with platin and paclitaxel (abstract). Proc Am Soc Clin Oncol 20: 216a
Shi LM, Myers TG, Fan Y, O'Connor PM, Paull KD, Friend SH, Weinstein JN (1998) Mining the National Cancer Institute anticancer drug discovery database: cluster analysis of ellipticine analogs with p53-invese and central nervous system-selective patterns of activity. Mol Pharmacol 53: 241–251
Stupp R, Bodmer A, Duvoisin B, Bauer J, Perey L, Bakr M, Ketterer N, Leyvraz S (2001) Is cisplatin required for the treatment of non-small-cell lung cancer. Experience and preliminary results of a phase I/II trial with topotecan and vinorelbine. Oncology 61(Suppl. 1): 35–41
Su Y-Z, Sommers B, Horlick D, Harris C, Evans S, Nagourney R (2001) Synergy between topotecan and other cytotoxic drugs in human tumors (abstract). Proc Am Assoc Cancer Res 42: 253–254
Taguchi T, Tominaga T, Ogawa M, Ishida T, Morimoto K, Ogawa N (1994) A late phase II study of CPT-11 (irinotecan) in advanced breast cancer. CPT-11 Study Group on Breast Cancer). Gan To Kagaku Ryoho 21: 1017–1024
ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, Broom C, Scarabelli C, Davidson N, Spanczynski M, Bolis G, Malmström H, Coleman R, Fields SC, Heron JF (1997) Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol 15: 2183–2193
The Elderly Lung Cancer Vinorelbine Italian Study Group (1999) Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 91: 66–72
Verweij J, Lund B, Beijnen J, Planting A, de Boer-Dennert M, Koier I, Rosing H, Hansen H (1993) Phase I and pharmacokinetics study of topotecan, a new topoisomerase I inhibitor. Ann Oncol 4: 673–678
von Pawel J, Gatzemeier U, Pujol JL, Moreau L, Bildat S, Ranson M, Richardson G, Steppert C, Riviere A, Camlett I, Lane S, Ross G (2001) Phase II comparator study of oral versus intravenous topotecan in patients with chemosensitive small-cell lung cancer. J Clin Oncol 19: 1743–1749
von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A, Carmichael J, Krebs JB, Ross G, Lane SR, Gralla R (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17: 658–667
Wall JG, Burris III HA, Von Hoff DD, Rodriguez G, Kneuper-Hall R, Shaffer D, O'Rourke T, Brown T, Weiss G, Clark G, McVea S, Brown J, Johnson R, Friedman C, Smith B, Mann WS, Kuhn J (1992) A phase I clinical and pharmacokinetic study of the topoisomerase I inhibitor topotecan (SK&F 104864) given as an intravenous bolus every 21 days. Anticancer Drugs 3: 337–345
Weitz JJ, Marschke Jr RF, Sloan JA, Grill JP, Jett JR, Knost JA, Hatfield AK, Zenk DW, Bate WW, Schaefer PL (2000) A randomized phase II trial of two schedules of topotecan for the treatment of advanced stage non-small cell lung cancer. Lung Cancer 28: 157–162
Wiesenfeld M, Marks R, Grill J, Sloan J, Tazelaar H, Carroll T, Kugler J, Krook J (1997) A randomized phase II study of topotecan (TOPA) and cisplatin (CDDP) with filgrastim (G-CSF) versus topotecan and paclitaxel (Taxol®) with filgrastim in patients with advanced non-small cell lung cancer (abstract). Proc Am Soc Clin Oncol 16: 488a
Acknowledgements
We acknowledge the technical assistance of Craig Chou, Yong Zhuang Su, Cheryl Kollin Dorothy Horlick. This work was supported by GlaxoSmithKline, Philadelphia, PA, USA. We gratefully acknowledge the financial support of GlaxoSmithKline (Philadelphia, PA, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Nagourney, R., Sommers, B., Harper, S. et al. Ex vivo analysis of topotecan: advancing the application of laboratory-based clinical therapeutics. Br J Cancer 89, 1789–1795 (2003). https://doi.org/10.1038/sj.bjc.6601336
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6601336
Keywords
This article is cited by
-
A Deep Neural Network for Predicting Synergistic Drug Combinations on Cancer
Interdisciplinary Sciences: Computational Life Sciences (2024)
-
5-Flurouracil disrupts nuclear export and nuclear pore permeability in a calcium dependent manner
Apoptosis (2017)
-
Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment
BMC Cancer (2006)
-
Ex vivo programmed cell death and the prediction of response to chemotherapy
Current Treatment Options in Oncology (2006)
-
Augmenting tumor sensitivity to topotecan by transient hypoxia
Cancer Chemotherapy and Pharmacology (2005)