Abstract
Large primary breast tumours and extensive lymph node involvement are linked to a high rate of local recurrence after surgery. In 10–20% of such high-risk breast cancer patients, local relapse will occur despite postoperative radiotherapy. In the present study, we investigated whether molecular features, such as angiogenesis, cancer cell proliferation, steroid receptor expression, c-erbB-2 oncoprotein overexpression, p53 protein nuclear accumulation or bcl-2 antiapoptotic protein expression, can predict failure of local therapy. We further examined as to which subgroups of patients could benefit from altered fractionation schemes of radiotherapy. In univariate analysis, high intratumoural angiogenesis, c-erbB overxpression and mutant-p53 nuclear accumulation were significantly associated with increased relapse rate (P=0.0002, 0.009 and 0.05, respectively). In multivariate analysis, the microvessel density and the c-erbB-2 status were independent and significant factors related to local relapse (P=0.001, t-ratio 3.36 and P=0.02, t-ratio 2.26, respectively). Hypofractionated and accelerated radiotherapy supported with amifostine (HypoARC regimen) was significantly more effective than standard radiotherapy in cases with high cancer cell proliferation index, c-erbB-2 and p53 overexpression. High angiogenesis, however, was linked with local relapse regardless of the radiotherapy regimen.
Similar content being viewed by others
Main
Adjuvant radiotherapy significantly improves the local control rates even after radical or partial mastectomy in patients with aggressive tumour features, such as large primary tumours or extensive node involvement (Forrest et al, 1996; Early Breast Cancer Trialists Collaborative Group, 1995; Griem et al, 1987; Velez-Garcia et al, 1992). Although controversial, several large studies suggested that local control is also important for the overall survival of patients (Overgaard et al, 1997, 1999; Ragaz et al, 1997; Clark et al, 1996). As local recurrence after surgery (with or without chemotherapy) is less than 20% (Mcneil, 1999; Recht et al, 1999), radiotherapy is often omitted (Athas et al, 2000) as it is difficult to weigh the benefits of reduced cost and patient inconvenience that result from the elimination of postoperative radiotherapy against the disadvantages of local recurrence (Liljegren et al, 1994).
A recent study reported the results of a prospective study on 72 breast cancer patients with features of locally aggressive or advanced disease treated with surgery, FEC (5-fluorouracil 500 mg m−2, epirubicine 60 mg m−2 and cyclophosphamide 500 mg m−2) chemotherapy and a very accelerated scheme of hypofractionated radiotherapy supported with high-dose daily amifostine (Koukourakis et al, 2002a). The whole radiotherapy dose is given within 12 consecutive radiotherapy fractions (16 days). After a median of 28 months of follow-up (range 18–42 months), we concluded that the regimen is well tolerated with significantly reduced early radiation sequelae and with late toxicity (breast, axilla, thoracic wall) at least equal to that observed in control groups of patients, within the same follow-up time.
Such a regimen is certainly convenient for patients and radiotherapy departments, as it reduces the overall days of radiotherapy down to 35% of the days required for a 45-day conventional fractionation regimen. Nevertheless, the rationale for applying such an aggressive regimen was also based on a potential advantage in terms of local control. Hypofractionated radiotherapy regimen supported with amifostine cytoprotection (HypoARC), theoretically, could be more effective for tumours for several reasons described in detail in previous papers (Koukourakis et al, 2002a; Koukourakis and Yannakakis, 2001). Briefly, radiotherapy acceleration could abrogate the ominous effect of ‘rapid tumour repopulation’ on the efficacy of radiotherapy. Furthermore, the use of large radiotherapy fractions could prove more effective in tumours with low intrinsic radiosensitivity. As hypoxia pushes the β-value of the α/β ratio to higher levels (Chapman et al, 1975), hypoxic tumours may be resistant to low dose per fraction used in standard or hyperfractionated radiotherapy. In that way, HypoARC could be more effective in hypoxic breast tumours. On the other hand, the addition of high-dose amifostine before each radiotherapy fraction could help in overcoming the severe early and late toxicity expected from such an aggressive radiotherapy regimen, which is indeed confirmed in a prospective study (Koukourakis et al, 2002a). Local relapse analysis, however, did not show a significant benefit over standard radiotherapy, although the 1- and 2-year local relapse-free survival (LRFS) was 95 and 84% in the HypoARC group, respectively, vs 84 and 76% in the standard radiotherapy arm (Koukourakis et al, 2002a).
In the present study, we show that specific molecular features relevant to rapid tumour repopulation or to intrinsic radiosensitivity (i.e. high proliferation index, p53-mutations or c-erbB-2 overexpression) are linked with high local relapse rate following postoperative radiotherapy, which can be averted by using large radiotherapy fractions and treatment acceleration. High angiogenesis, however, predicts a high rate of local relapse independent of the radiotherapy scheme.
Materials and methods
From March 1997 to April 1999 at the University Hospital of Heraklion, 72 high-risk breast cancer patients treated with surgery and adjuvant FEC chemotherapy were recruited in a large phase II study in order to assess the toxicity and efficacy of HypoARC as an adjuvant regimen (Koukourakis et al, 2002a). Inclusion and exclusion criteria have been previously reported. Briefly, high-risk breast cancer patients were recruited (T3,4-stage and/or metastatic disease in more than three lymph nodes), and all patients had a good performance status with no other major disease. Informed consent was obtained before inclusion.
The survival of patients treated with adjuvant HypoARC chemotherapy was compared to that of a matched control group of 59 breast cancer patients treated with modified radical mastectomy followed by six cycles of FEC chemotherapy and conventionally fractionated radiotherapy. These patients were treated at the same department, during the same period and fulfilled the criteria used for the recruitment of patients in the HypoARC study. Patients' characteristics and tumour stage were similar in both groups (Table 1). All tumours were of the ductal type, unifocal and the surgical margins were negative as revealed in the histopathology report.
Archieval paraffin-embedded material from the primary breast carcinoma was retrieved from hospitals, where the surgery had been performed. We managed to collect tissue samples from 31 out of 72 patients treated with HypoARC and from 40 out of 59 patients treated with conventional radiotherapy (CRT). Failure to collect additional samples was mainly because of pathological diagnosis in centres outside the island of Crete and because of exhausted tissue material (used in other research projects).
Radiotherapy details
The same irradiation technique was used for standard radiotherapy and HypoARC. Field positioning was performed with the aid of a simulator following computerised planning. The breast (or chest wall), axilla and supraclavicular area were treated with a LINAC 6MV. Tangential fields were used to irradiate the breast or chest wall and special care was taken during the simulation so that less than 2 cm of thickness of normal lung was included in these fields. The inner borders were placed 2 cm away from the sternal midline while the outer border followed the middle axillary line. Supraclavicular and axillary areas were irradiated with an anterior field, the dose being calculated at 4 cm of depth. A booster posterior field directed to the axilla (dose calculation at 7 cm) was treated immediately following the treatment of the anterior field in order to compensate for the loss of dose because of increased depth. The dose administered with this posterior field was 15% of the dose given with the anterior one. Tangential fields were also used to deliver a booster dose to the tumour bed, where a 1 cm tissue equivalent material was placed over this area (5–8 cm × 7–10 cm) in order to increase the dose to the surface. The onset of radiotherapy was 25–30 days after the delivery of the last cycle of chemotherapy. Table 2 shows the radiotherapy fractionation used. Radiobiological analysis has been reported in a previous study (Koukourakis et al, 2002a).
Administration of amifostine
Tropisetron (10 mg i.v., in 50 ml NS infused within 5 min) was given as antiemetic premedication. Amifostine (Ethyol®), 1000 mg diluted in 50 ml NS, was rapidly infused within 5 min, the patient being in a supine position, and under continuous monitoring of blood pressure. No steroids were used unless indicated by severe vomiting. Radiotherapy was delivered 20 min after the amifostine infusion.
Immunohistochemical studies
A modified streptavidin immunohistochemical technique was used to assess the expression of different proteins and oncoproteins. Sections were dewaxed and peroxidase was quenched with methanol and H2O2 3% for 15 min. Microwaving for antigen retrieval was used (3 × 4 min). The primary antibodies were applied for 90 min. Following washing with TBS, sections were incubated with a secondary rabbit-anti-mouse antibody (Kwik Biotinylated Secondary, 0.69A Shandon-Upshaw) for 15 min and washed in TBS. Kwik Streptavidin peroxidase reagent (039A Shandon-Upshaw) was applied for 15 min and sections were again washed in TBS. The colour was developed by 15 min incubation with DAB solution and sections were weakly counterstained with haematoxylin. Normal mouse immunoglobulin-G was substituted for primary antibody as the negative control (same concentration as the test antibody), while appropriate tissue samples were used for positive controls.
For the assessment of the proliferation index, the MIB-1 MoAb (clone PRO224, YLEM, Italy) was used. The percentage of cells with nuclear reactivity was recorded in all fields and the mean value was calculated. The median MIB-1 score was used as a cutoff point to define two groups of tumours with low vs high proliferation index.
For c-erbB-2 expression, the NCL-CB11 MoAb was used (Novocastra Laboratories, UK). The percentage of cancer cells with a clear membrane staining was recorded in all optical fields and the mean value was calculated. Tissue samples with a mean value >20% were considered as positive for c-erbB-2 protein overexpression.
For mutant p53 protein expression, we used the DO7 MoAb (DAKO, Denmark). The percentage of cancer cells with nuclear reactivity was recorded in all optical fields and the mean value was calculated. Tumour samples with a percentage of positive cancer cells >10% were considered as positive for p53. Using a similar method of assessment, the nuclear expression of oestrogen and progesterone receptors was examined by applying the mouse MoAb clone 1D5 and the clone 1A6 (dilution 1 : 100; Immunon-Shandon, USA), respectively. A cutoff point of 10% of reactive cells was also used for positivity.
For bcl-2 protein expression, the clone 100 MoAb (DAKO, Denmark) was used. The percentage of cancer cells with strong cytoplasmic and/or perinuclear expression was recorded in all optical fields and the mean value was calculated. Tumour samples with a mean value higher than 10% were considered as positive for bcl-2 expression.
Assessment of the intratumoural microvessel density (MVD)
The JC70 monoclonal antibody (DAKO, Denmark) recognising the CD31 (platelet/endothelial cell adhesion molecule; PECAM-1) was used for microvessel staining on 2 μm paraffin-embedded sections using the alkaline phosphatase/antialkaline phosphatase (APAAP) procedure. Sections were dewaxed, rehydrated and predigested with protease type XXIV for 20 min at 37°C. The JC70 (1 : 20) was applied at room temperature for 30 min and washed in TBS. Rabbit-anti-mouse antibody 1 in 50 was applied for 30 min, followed by application of APAAP complex 1 in 1 for 30 min. After washing in TBS, the last two steps were repeated for 10 min each. The colour was developed by 20 min incubation with New Fuchsin solution. Sections from primary tumours were scanned at low power (×40 and ×100).
The areas of the highest vascularisation were chosen at low power (×100) and microvessel counting followed on three chosen ×200 fields of the highest density (hot spots). Microvessels adjacent to normal breast or around necrotic areas were excluded from the appraisal. The MVD was the mean of the vessel counts obtained. The median MVD was used to define two groups of patients with low vs high MVD. Vessels with a clearly defined lumen or well-defined linear vessel shape but not single endothelial cells were taken into account for microvessel counting.
Statistical analysis
Statistical analysis and graphs were performed using the GraphPad Prism 2.01 package. A Fisher's exact test or the unpaired two-tailed t-test was used for testing relations between the categorical variables, as appropriate. Local relapse and overall curves were plotted using the method of Kaplan–Meier, and the log-rank test was used to determine statistical differences between life tables. A Cox proportional hazard model was used to assess the effects of patient and tumour variables on overall survival. A P-value of <0.05 was considered significant.
Results
Results of immunohistochemistry
The results of immunohistochemistry in the two groups of patients (HypoARC vs CRT) are shown in Table 3. Overall, 35 out of 71 patients had high intratumoural MVD, 24 out of 71 high MIB-1 proliferation index, 17 out of 71 had membrane c-erbB-2 protein overexpression, 21 out of 71 had nuclear mt-p53 protein accumulation and 45 out of 71 had positive cytoplasmic bcl-2 expression. Oestrogen and progesterone receptor expression in the cancer cell nuclei was noted in 42 out of 71 and 21 out of 71 tumours, respectively. The immunohistochemical findings were well balanced between the two groups, but the bcl-2 expression was more frequently noted in the group of patients treated with HypoARC.
Local relapse-free survival (LRFS) analysis
Out of 31 patients treated with HypoARC, four (12.9%) relapsed locally vs seven out of 40 (17.6%) of patients treated with CRT. The LRFS of patients treated with HypoARC or CRT is plotted in Figure 1. There was no significant difference between the two groups (P=0.59). The type of surgery (mastectomy vs tumourectomy) or the menopausal status was not related to local relapse (P=0.40 and 0.96, respectively; data not shown).
Table 4 shows the univariate and multivariate analyses of LRFS in all patients considered together. In univariate analysis, high intratumoural angiogenesis, c-erbB overxpression and mutant-p53 nuclear accumulation were significantly associated with increased relapse rate (P=0.0002, 0.009 and 0.05, respectively). In multivariate analysis, the MVD and the c-erbB-2 status were independent and significant factors related to local relapse (P=0.001, t-ratio 3.36 and, P=0.02, t-ratio 2.26, respectively).
Local relapse-free survival LRFS analysis according to the radiotherapy regimen
The Kaplan–Meier LRFS curves were plotted and the log-rank test was calculated, using double stratification for the radiotherapy method and for each one of the assessed histological and molecular variables.
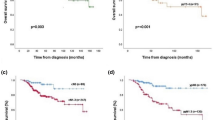
High MVD was associated with poor LRFS whether patients were treated with HypoARC or CRT (Figure 2A). On the contrary, high proliferation MIB-1 index was associated with significantly poor LRFS in patients treated with CRT (P=0.007), but not in patients treated with HypoARC (P=0.50). Patients with high proliferation index treated with HypoARC had a significantly better LRFS than patients treated with CRT (P=0.04); Figure 2B.
(A) Kaplan–Meier survival curves stratified for radiotherapy schedule (conventional vs HypoARC) and microvessel density (low vs high). (B) Kaplan-Meier survival curves stratified for radiotherapy schedule (conventional vs HypoARC) and MIB1 proliferation index (low vs high). (C) Kaplan–Meier survival curves stratified for radiotherapy schedule (conventional vs HypoARC) and c-erbB-2 membrane overexpression (negative vs positive).
Similarly, patients overexpressing c-erbB-2 had a significantly poorer LRFS when treated with CRT (P=0.001), while c-erbB-2 status was not of predictive significance in patients treated with HypoARC. The LRFS of c-erbB-2+ patients treated with HypoARC was better, although not significantly, than the one of c-erbB-2+ patients treated with CRT (P=0.13); Figure 2C.
Nuclear expression of mt-p53 was associated with poor LRFS only in the group of patients treated with CRT (P=0.002). Patients with mt-p53+ tumours treated with HypoARC had a better LRFS than patients with mt-p53+ tumours treated with CRT (P=0.06).
Analysis of T-stage, N-stage and histology grade did not reveal any significant differences between the HypoARC and the CRT efficacy in preventing local relapse. Similarly, HypoARC and CRT were equally effective within the positive or negative for ER, PgR or bcl-2 subgroups of patients (data not shown).
Multivariate analysis within the group of patients treated with CRT revealed that high MVD (P=0.009, t-ratio 2.76), c-erbB-2 overexpression (P=0.05, t-ratio 1.98) and mt-p53 expression (P=0.05, t-ratio 1.96) were independent variables related to local relapse. Multivariate analysis within the HypoARC group of patients showed that only high MVD had an independent predictive role of local relapse, although of marginal significance (P=0.07, t-ratio 1.86).
Discussion
An important role of molecular variables in defing relapse of breast cancer after local and systemic therapy has been raised during the past decade. Loss of oestrogen or progesterone receptor expression was the first molecular feature recognised to associate with poor survival in breast cancer (Chevallier et al, 1988). C-erbB-2 expression has been linked to resistance to chemotherapy and poor outcome (Andrulis et al, 1998), while apoptosis-related proteins such as p53 and bcl-2 have been related to disease-free interval and survival of breast cancer patients (Thor et al, 1992; Gasparini et al, 1995). Similarly, a large body of clinicopathological studies support the important and independent prognostic role of the tumour angiogenic ability in operable breast cancer (Locopo et al, 1998). Most of the studies, however, focus on the overall relapse events, while the impact of molecular variables on the efficacy of postoperative radiotherapy to control local disease remains largely unknown.
Nevertheless, several histopathological or patient-related variables have been reported to define a higher risk of local relapse after postoperative radiotherapy. In a recent analysis by Katz et al (2001), in 1031 mastectomised breast cancer patients, disease close to the surgical margins, gross multifocality, tumour size >5 cm and cancer metastasis in more than three axillary nodes were independently related to a higher risk of local relapse following postoperative radiotherapy. Similar results have been reported in another recent study from Japan, in 301 stage I/II breast-cancer patients treated with lumpectomy or quadrantectomy (Kodaira et al, 2001). Young age and less extensive surgery had an independent predictive role of local relapse, while positive surgical margins or extensive intraductal component had a marginal significance. Systemic therapy seems also to contribute to the local relapse-free interval of breast-cancer patients, as recently shown in a study of 484 node negative breast cancer patients treated with breast conservative surgery and radiotherapy (Buchholz et al, 2001).
Our study comprised a relatively limited number of breast cancer patients. All of them, however, although operable had local and/or regional extensive disease. This was a result of the inclusion criteria used for the enrolment of patients in the HypoARC study (Koukourakis et al, 2002a). All patients had received FEC chemotherapy before radiotherapy. Clear surgical margins and unifocal disease was a prerequisite to include a tumour sample in the present clinicopathological study. Thus, out of the traditional patient/histology variables, menopausal status and surgical technique were the only ones left to test as predictors of local relapse. None of them was significantly related to local relapse in this high-risk series of patients.
Molecular analysis, however, showed that increased intratumoural MVD, c-erbB-2 membrane overexpression, accumulation of mutant p53 protein and high proliferation index assessed with the MIB-1 MoAb defines subgroups of patients with increased risk of local relapse, in high-risk breast cancer patients. In multivariate analysis, high MVD and c-erbB-2 overexpression retained their significant and independent predictive role for local relapse, while p53 and proliferation index did not.
The important role of upregulated angiogenic pathways in the outcome of radiotherapy has been recently raised (Koukourakis et al, 2000a, 2000b). It seems that high angiogenic activity confers a proliferation/apoptosis advantage in cancer cells during radiotherapy (Matsushita et al, 1999; Natsura et al, 1999), while an angiogenic regeneration of tumours during radiotherapy cannot be excluded (Koukourakis et al, 2000b). C-erbB-2 protein overexpression in breast cancer seems also to relate to resistance to radiotherapy (Formenti et al, 2002). Treatment of cancer cells with anti-c-erbB-2 MoAbs before radiotherapy significantly enhances the cytotoxic effect of radiotherapy, which shows that c-erbB-2 overexpression is involved in biological pathways relevant to the intrinsic radiosensitivity (Pietras et al, 1999). c-erbB-2 involvement in the upregulation of the VEGF angiogenic pathway (Petit et al, 1997) suggests that c-erbB-2-related radioresistance is, at least in part, linked to the tumour angiogenic activity. The involvement of wild-type p53 protein in the radiation-induced apoptosis (Brugarolas et al, 1995), at least in part, explains the finding that mutant p53 expression is related to reduced efficacy of local radiotherapy. A strong association of nuclear p53 protein accumulation with local relapse in breast cancer patients receiving or not postoperative radiotherapy has been recently reported by Zellars et al (2000). The association of a high proliferation index with local relapse may be explained by a possible role of rapidly proliferating cells in the so-called ‘accelerated clonogen repopulation’ phenomenon during radiotherapy (Withers et al, 1998). An increased rate of locoregional and distant relapse has been reported in patients with breast cancer bearing a high thymidine labelling index (Amadori and Silvestrini, 1998).
About half of the cases included in the study were treated with an accelerated HypoARC. Analysis of local relapse within the groups of patients treated with CRT and HypoARC radiotherapy showed a striking difference regarding the influence of the examined molecular variables. High proliferation index was not a predictive factor of local relapse in the group of patients treated with accelerated (HypoARC) radiotherapy. The 2-year LRFS was 100% in the HypoARC group vs 56% in patients treated with CRT. This shows that radiotherapy acceleration overcomes the ominus effect of a high cancer cell proliferation rate, which is in accordance with the concept that accelerated radiotherapy overcomes the effect of ‘accelerated repopulation’.
Similarly, HypoARC was more effective than CRT in tumours with p53 mutations. The 2-year LRFS was 100% in the HypoARC group vs 54% in the CRT group. Loss of wild-type p53 protein results in failure of radiation-induced DNA damage to trigger apoptosis events downstream p53 (Brugarolas et al, 1995). Large radiotherapy fractions that induce a more severe DNA damage may be more effective in wt-p53-deficient cells, which could explain the higher efficacy of HypoARC. HypoARC was also more effective in c-erbB-2 overexpressing tumours. Although a definite explanation for this finding cannot be provided, acceleration of radiotherapy may have overcome an eventual growth advantage conferred by c-erbB-2 upregulation (Mandler et al, 2000). Still, recent studies suggest that c-erbB-2-is involved in the repair of the radiation-induced DNA damage as MoAbs against c-erbB-2 enhance radiosensitivity of human breast and head–neck cancer cells (Pietras et al, 1999; Uno et al, 2001). Hypofractionation could therefore contribute to overcome an eventual c-erbB-2-dependent intrinsic radioresistance.
An interesting finding was that highly angiogenic tumours were resistant to radiotherapy, whether conventionally fractionated, accelerated or hypofractionated. This shows that altered fractionation or reduced overall treatment time are impossible to counteract the radioresistance pathways related to tumour angiogenicity. Antiangiogenic policies are awaited to improve the local control in patients with highly angiogenic breast cancer. Indeed, several experimental data suggest an important enhancement of the efficacy of radiotherapy when combined with various antiangiogenic agents (Gorski et al, 1999; Mauceri et al, 1998).
In a previous study, we showed that HypoARC is a regimen with reduced early toxicity and, at least within a median of 28 months of follow-up, late radiation sequelae were in the range of that expected from CRT. In the present study, we showed that HypoARC regimen is not at all equivalent to CRT, in terms of antitumour activity. It is evident that HypoARC, by using large radiotherapy fractions while accelerating radiotherapy, targets subgroups of breast tumours with a high risk to relapse following CRT. Tumours with a high proliferation index and eventually a high fraction of clonogens able to enter the phase of accelerated repopulation during CRT, as well as tumours with increased intrinsic radioresistance (e.g. mutations of p53 or c-erbB-2 gene amplification) have about 50% chance to recure after CRT; this seems to be averted using HypoARC. The ominous impact of upregulated angiogenic pathways, however, is unlikely to be prevented by altering the radiotherapy schedules. Antiangiogenic policies are demanded to improve local control. As HypoARC seems to overcome both intrinsic radioresistance and accelerated repopulation, taking also into account the promising low early and late side effects of the regimen, its role in clinical radiotherapy should be thoroughly examined. Prostate cancer, recently shown to have an α/β ratio lower than 2 Gy (Fowler et al, 2001), and also tumours with low intrinsic radiosensitivity (e.g. sarcomas, melanomas and pancreatic carcinoma) are expected to benefit from HypoARC regimens. The above findings should, however, be confirmed in larger studies, once mature data from ongoing HypoARC studies become available.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amadori D, Silvestrini R (1998) Prognostic and predictive value of thymidine labelling index in breast cancer. Breast Cancer Res Treat 51: 267–281
Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L, Wilkinson R, Qizilbash A, Ambus U, Lipa M, Weizel H, Katz A, Baida M, Mariz S, Stoik G, Dacamara P, Strongitharm D, Geddie W, McCready D (1998) neu/erbB-2 Amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol 16: 1340–1349
Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR (2000) Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 92: 269–271
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557
Buchholz TA, Tucker SL, Erwin J, Mathur D, Strom EA, McNeese MD, Hortobagyi GN, Cristofanilli M, Esteva FJ, Newman L, Singletary E, Buzdar AU, Junt KK (2001) Impact of systemic treatment on local control for patients with lymph node-negative breast cancer treated with breast-conservation therapy. J Clin Oncol 19: 2240–2246
Chapman JD, Gillespie CJ, Reuvers AP, Dugle DL (1975) The inactivation of Chinese hamster cells by X-rays: the effect of chemical modifiers on single and double-events. Radiat Res 64: 365–375
Chevallier B, Heintzmann F, Mosseri V, Dauce JP, Bastit P, Graic Y, Brunelle P, Basuyau JP, Comoz M, Asselain B (1988) Prognostic value of estrogen and progesterone receptors in operable breast cancer. Results of a univariate and multivariate analysis. Cancer 62: 2517–2524
Clark RM, Whelan T, Levine M, Roberts R, Willan A, McCulloch P, Lipa M, Wilkinson RH, Mahoney LJ (1996) Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. Ontario Clinical Oncology Group. J Natl Cancer Inst 88: 1659–1664
Early Breast Cancer Trialists' Collaborative Group (1995) Effects of radiotherapy and surgery in early breast cancer: an overview of the randomized trials. N Engl J Med 333: 1444–1455
Formenti SC, Spicer D, Skinner K, Cohen D, Groshen S, Bettini A, Naritoku W, Press M, Salonga D, Tsao-Wei D, Danenberg K, Danenberg P (2002) Low HER2/neu gene expression is associated with pathological response to concurrent paclitaxel and radiation therapy in locally advanced breast cancer. Int J Radiat Oncol Biol Phys 52: 397–405
Forrest AP, Stewart HJ, Everington D, Prescott RJ, McArdle CS, Harnett AN, Smith DC, George WD (1996) Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Scottish Cancer Trials Breast Group. Lancet 348: 708–713
Fowler J, Chappell R, Ritter M (2001) Is α/β for prostate tumours really low? Int J Radiat Oncol Biol Phys 50: 1021–1031
Gasparini G, Barbareschi M, Doglioni C, Palma PD, Mauri FA, Boracchi P, Bevilacqua P, Caffo O, Morelli L, Verderio P (1995) Expression of bcl-2 protein predicts efficacy of adjuvant treatments in operable node-positive breast cancer. Clin Cancer Res 1: 189–198
Griem KL, Henderson IC, Gelman R, Ascoli D, Silver B, Recht A, Goodman RL, Hellman S, Harris JR (1987) The 5-year results of a randomized trial of adjuvant radiation therapy after chemotherapy in breast cancer patients treated with mastectomy. J Clin Oncol 5: 1546–1555
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR (1999) Blockage of the vascular endothelial growth factor stress response increases the antitumour effects of ionizing radiation. Cancer Res 59: 3374–3378
Katz A, Strom EA, Buchholz TA, Theriault R, Singletary E, McNeese MD (2001) The influence of pathologic tumour characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys 50: 735–742
Kodaira T, Fuwa N, Itoh Y, Matsumoto A, Kamata M, Furutani K, Sasaoka M, Miura S, Tajeuchi T (2001) Aichi cancer center 10-year experience with conservative breast treatment of early breast cancer: retrospective analysis regarding failure patterns and factors influencing local control. Int J Radiat Oncol Biol Phys 49: 1311–1316
Koukourakis MI (2002b) Tumour angiogenesis and response to radiotherapy. Anticancer Res 21: 4285–4300.
Koukourakis MI, Giatromanolaki A, Fountzilas G, Sivridis E, Gatter KC, Harris AL (2000a) Angiogenesis, thymidine phosphorylase and resistance of squamous cell head and neck cancer to cytotoxic and radiation therapy. Clin Cancer Res 6: 381–389
Koukourakis MI, Giatromanolaki A, Kouroussis C, Kakolyris S, Sivridis E, Frangiadaki C, Retalis G, Georgoulias V (2002a) Hypofractionated and accelerated radiotherapy with cytoprotection (HypoARC): a short, safe, and effective postoperative regimen for high-risk breast cancer patients. Int J Radiat Oncol Biol Phys 52: 144–155
Koukourakis MI, Giatromanolaki A, Sivridis E, Fezoulidis I (2000b) Cancer vascularization: implications in radiotherapy? Int J Radiat Oncol Biol Phys 48: 545–553
Koukourakis MI, Yannakakis D (2001) High dose daily amifostine and hypofractionated intensively accelerated radiotherapy for locally advanced breast cancer. A phase I/II study and report on early and late sequellae. Anticancer Res 21: 2973–2978
Liljegren G, Holmberg L, Adami HO, Westman G, Graffman S, Bergh J (1994) Sector resection with or without postoperative radiotherapy for stage I breast cancer: five-year results of a randomized trial. Uppsala-Orebro Breast Cancer Study Group. J Natl Cancer Inst 86: 717–722
Locopo N, Fanelli M, Gasparini G (1998) Clinical significance of angiogenic factors in breast cancer. Breast Cancer Res Treat 52:159–173
Mandler R, Wu C, Sausville EA, Roettinger AJ, Newman DJ, Ho DK, King CR, Yang D, Lippman ME, Landolfi NF, Dadachova E, Brechbiel MW, Waldmann TA (2000) Immunoconjugates of geldamycin and anti-HER2 monoclonal antobodies: antiproliferative activity on human breast carcinoma cell lines. J Natl Cancer Inst 92: 1549–1551
Matsushita S, Nitanda T, Furukawa T, Sumizawa T, Tani A, Nishimoto K, Akiba S, Miyadera K, Fukushima M, Yamada Y, Yoshida H, Kanzaki T, Akiyama S (1999) The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumours. Cancer Res 59: 1911–1916
Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, Bigelow K, Heimann R, Gately S, Dhanabal M, Soff GA, Sukhatme VP, Kufe DW, Weichselbaum RR (1998) Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 1394: 287–291
Mcneil C (1999) Postmastectomy radiation: back to center stage? J Natl Cancer Inst 3: 1800
Natsura T, Kuratate I, Teramchi K, Osaki M, Fukuda Y, Ito H (1999) Thymidine phosphorylase expression is associated with both increase of intratumoral microvessels and decrease of apoptosis in human colorectal carcinomas. Cancer Res 59: 5037–5040
Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337: 949–955
Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353: 1641–1648
Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS (1997) Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumour cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumours. Am J Pathol 151: 1523–1530
Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ (1999) Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 59: 1347–1355
Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ, Basco VE, Wilson KS, Knowling MA, Coppin CM, Paradis M, Coldman AJ, Olivotto IA (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337: 956–962
Recht A, Gray R, Davidson NE, Fowble BL, Solin LJ, Cummings FJ, Falkson G, Falkson HC, Taylor SG, Tormey DC (1999) Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: experience of the Eastern Cooperative Oncology Group. J Clin Oncol 17: 1689–1700
Thor AD, Moore DH II, Edgerton SM, Kawasaki ES, Reihsaus E, Lynch HT, Marcus JN, Schwartz L, Chen LC, Mayall BH (1992) Accumulation of p53 tumour suppressor gene protein: an independent marker of prognosis in breast cancers. J Natl Cancer Inst 84: 845–855
Uno M, Otsuki T, Kurebayashi J, Sakaguchi H, Isozaki Y, Ueki A, Yata K, Fujii T, Hiratsuka J, Akisada T, Harada T, Imajo Y (2001) Anti-HER2-antibody enhances irradiation-induced growth inhibition in head and neck carcinoma. Int J Cancer 94: 474–479
Velez-Garcia E, Carpenter Jr JT, Moore M, Vogel CL, Marcial V, Ketcham A, Singh KP, Bass D, Bartolucci AA, Smalley R (1992) Postsurgical adjuvant chemotherapy with or without radiotherapy in women with breast cancer and positive axillary nodes: a South-Eastern Cancer Study Group (SEG) Trial. Eur J Cancer 28: 1833–1837
Withers HR, Taylor JMG, Maciejewski B (1998) The hazards of accelerated tumour clonogene repopulation during radiotherapy. Acta Oncol 27: 131–146
Zellars RC, Hilsenbeck SG, Clark GM, Allred DC, Herman TS, Chamness GC, Elledge RM (2000) Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J Clin Oncol 18: 1906–1913
Acknowledgements
The study was financially supported by the Tumour and Angiogenesis Research Group.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Koukourakis, M., Giatromanolaki, A., Galazios, G. et al. Molecular analysis of local relapse in high-risk breast cancer patients: can radiotherapy fractionation and time factors make a difference?. Br J Cancer 88, 711–717 (2003). https://doi.org/10.1038/sj.bjc.6600755
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600755
Keywords
This article is cited by
-
Young age is associated with ipsilateral breast tumor recurrence after breast conserving surgery and radiation therapy in patients with HER2-positive/ER-negative subtype
Breast Cancer Research and Treatment (2011)
-
Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer
BMC Cancer (2008)