Abstract
In the present study, we investigated the association of the serum levels of the tumour markers carcinoembryonic antigen and cancer antigen 15-3 with disease free survival and death from disease in 1046 women with breast cancer without metastases at the time of primary diagnosis in relation to age and the established prognostic factors tumour size, lymph node status, histological grading and hormone receptor status. We found that elevated pre-operative serum marker values were correlated with early relapse (cancer antigen 15-3; P=0.0003) and death from disease (carcinoembryonic antigen, cancer antigen 15-3; P=0.0001 both) in univariate analyses. By comparing pre- and post-operative values we found a decline in values post-surgery. In those patients where marker levels of carcinoembryonic antigen decreased more than 33%, a significantly higher risk for relapse and death from disease (both P=0.0001) in univariate analyses was observed. In multivariate analysis this decrease of carcinoembryonic antigen proved to be an independent prognostic factor. The results for cancer antigen 15-3 were comparable to carcinoembryonic antigen in univariate analyses but showed no significance in multivariate analysis. In this study the post-operative decrease of the serum tumour marker carcinoembryonic antigen was a strong independent prognostic factor for disease free survival and death from disease in breast cancer patients.
Main
As adjuvant therapy for breast cancer has become more generally used, there seems to be a decreased need for prognostic factors. Nonetheless it remains a challenge to predict which patients are at greatest risk of relapse and thus may benefit most from adjuvant therapy (McGuire and Clark, 1992; Mansour et al, 1994). In addition to traditional prognostic factors in breast cancer such as tumour size, axillary lymph node status, histological grading and hormone receptor status (Clark et al, 1987; Chevallier et al, 1988; Carter et al, 1989; Nomura et al, 1992; Carriaga and Henson, 1995), newer parameters like Her-2/neu, cathepsin D or urokinase plasminogen activator (uPA) and plasminogen activator inhibitor 1 (PAI-1) are under consideration (Mansour et al, 1994; Schmitt et al, 1997). Circulating tumour markers as Carcinoembryonic Antigen (CEA) and Cancer Antigen 15-3 (CA 15-3) have become well established diagnostic tools as fast, non-invasive, reproducible and quantitative parameters in follow-up care and monitoring therapy of breast cancer patients. However, the potential role of these factors in prognosis has only been studied in a few investigations, which came to inconsistent conclusions (Tondini et al, 1989; Lamerz et al, 1993; Shering et al, 1998). The only multivariate analysis based on a large number of patients (Shering et al, 1998) investigated CA 15-3 alone.
In the present study we analysed the serum markers CEA and CA 15-3 at the time of primary intervention, and related levels of both markers to patient outcome using both univariate and multivariate analysis.
Materials and methods
Patients
All female primary breast cancer patients, who underwent surgery between September 1985 and June 1998 at the University Department of Obstetrics and Gynecology, Klinikum Grosshadern, Munich, were considered for inclusion in this retrospective study. Patients were excluded if any other malignancy was known from their past history or if staging investigations at the time of diagnosis revealed evidence of distant metastases. Furthermore, the first line treatment had to be surgery with curative intent, the pathological staging (tumour size and axillary lymph node status according to the pTpN classification) had to be known and tumour marker values of CEA and CA 15-3 had to be available at least within 30 days preceding surgery. A total of 1046 patients fulfilled these criteria. Also, histological grading, age and hormone receptor status were evaluated at the time of diagnosis.
Patients were treated with either modified radical mastectomy or lumpectomy and axillary node dissection with local radiotherapy and adjuvant systemic therapy if indicated, i.e. chemotherapy in node positive patients and hormone therapy in receptor positive patients. Regarding the course of disease, follow-up controls were performed with patient history, physical examination and laboratory tests including serum tumour markers CEA and CA 15-3, abdominal ultrasound, chest radiography, mammography and bone scan for detection of local or distant relapse. Additionally, computed tomography, MRI-tomography and other radiographs were carried out if necessary.
Patients with secondary contralateral breast cancer were excluded with respect to disease free survival. In 844 cases data regarding relapse were available. The median follow-up time for patients free of relapse at the time of analysis was 2.4 (range 0.2–12.7) years, and for those with relapse 1.8 (0.2–10.4) years. In 201 out of 844 patients recurrence of cancer occurred (first relapse: local recurrence n=68, distant metastases n=127, both n=6).
Regarding death from disease, out of 1046 patients 74 were lost to follow-up and the cause of death remained unclear in 21 cases. Nine hundred and fifty-one cases could be followed up, the number of women years was 3434. Ten patients died of causes other than cancer, and 95 women died from breast cancer. The median follow-up period for patients still alive or deceased from causes other than breast cancer was 3.0 (0.2–12.7) years and 2.9 (0.7–11.1) years for those deceased from breast cancer. The final data set is summarised in Table 1.
Marker analysis
The serum tumour markers CEA (IMx; MEIA, Abbott Laboratories, Chicago, IL, USA) and CA 15-3 (ES 700; Enzymun, Roche Diagnostics ((previously Boehringer Mannheim) Germany) were determined by automated test systems using sandwich ELISA assay kits. We investigated the values of the tumour markers CEA and CA 15-3 at the time of primary diagnosis. In addition to pre-operative values, tumour marker concentrations could be determined in a lower number of patients after primary therapy (see Table 1).
Statistics
Patients were grouped according to tumour size (pTis and pT1/pT2/pT3/pT4), lymph node status, histological grading, age (<50/⩾50 years), hormone receptor status (cut-off 15 fmol mg−1), the pre-operative tumour marker level of CEA and CA 15-3 and the post-operative decrease of the tumour marker. For multivariate analyses patients with tumour size pT3 and pT4 were grouped together, as well as nodal status N1 and N2.
To define cut-off values of pre-operative tumour markers, we chose the 95%-percentile for healthy individuals of 2.0 ng ml−1 for CEA and 25 U ml−1 for CA 15-3 (Stieber, 1996). In addition to the pre-operative value we considered the difference of tumour-free or baseline values from initial pre-operative values. As individual baseline value we chose the first tumour marker value when primary therapy, including adjuvant therapy, was completed. For patients without adjuvant therapy the lowest value within 6 months after primary surgery was chosen. The difference of the pre-operative and the individual baseline value in per cent was called the post-operative decrease (cut-off: −33%, corresponding to the 90%-percentile).
Univariate survival curves for disease free survival and death from disease were estimated by the method of Kaplan–Meier (Kaplan and Meier, 1958) and differences between groups in survival or relapse-free time were tested using the log-rank test. Multivariate Cox regression analysis (Cox, 1972) was performed to identify those parameters having an independent significant influence on DFS and DFD and to calculate the hazard ratios.
All parameters found to be significant in univariate analyses were entered into multivariate Cox regression model and were excluded if their P-value was greater than 0.05. Interactions between all variables were investigated and the assumption of proportional hazards was tested by including time dependent variables in the model. All statistical analyses were carried out using SAS statistic software (SAS Institute, Inc., Cary, USA).
Results
Characteristics of the 1046 patients are listed in Table 1. The median pre-operative CEA value was 1.2 ng ml−1 (range: <1–47.8; 25%/75%-percentile: 1.0/2.3). The median pre-operative CA 15-3 value was 17.3 U ml−1 (range 3–1686; 25%/75%-percentile: 12.7/23.3). As shown in Figure 1, only patients with N2 lymph node status had higher values. Post-operative tumour marker values were available in a smaller number of patients (CEA: n=871, CA 15-3: n=740). The median (range; 25/75% percentile) of the baseline values after surgery was 1.0 ng ml−1 (<1-15; 1.0/1.4) for CEA and 14.8 U ml−1 (3.5–120; 10.8/19.7) for CA 15-3 (Figure 1). For the post-operative decrease the respective values were 0% (−41-100; 0/23.1) for CEA and 12.2% (−43-94; 0/26.9) for CA 15-3. Tumour size, lymph node status, histological grading as well as hormone receptor status were found to be significant in univariate analysis. Age had no significant influence on disease free survival or death from disease. Elevated pre-operative values of CEA and CA 15-3 were associated with early death from disease (P=0.0001 for both markers), for relapse high levels of CA 15-3 were also significant (P=0.0003), whereas elevated values of CEA only showed borderline significance (P=0.064). Individual baseline values as defined above had no prognostic value with either disease free survival or death from disease, as end points.
A decrease of more than 33% of the CEA value from the pre-operative level was associated with early relapse and death from disease (both P=0.0001). A decrease of CA 15-3 of more than 33% was an indication of bad prognosis (death from disease P=0.007; disease free survival P=0.0087).
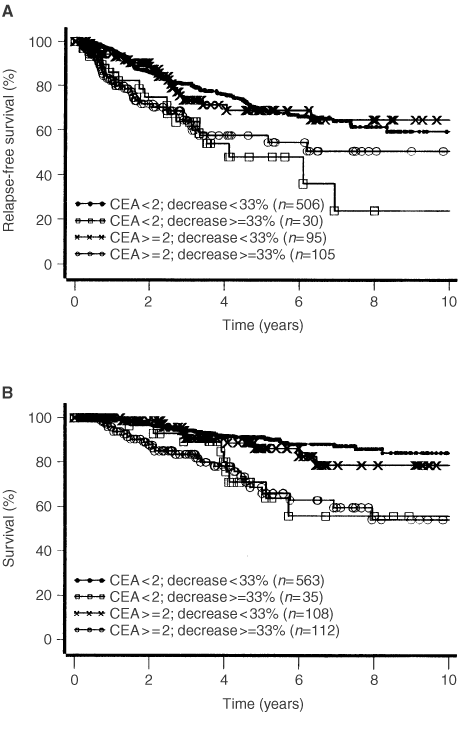
Kaplan–Meier curves for DFS and DFD are shown for the combination of pre-operative value and post-operative decrease of CEA and CA 15-3, respectively (Figures 2 and 3). Those patients whose elevated pre-operative values of CA 15-3 decreased more than 33% had the highest risk of relapse and death from cancer (Figure 2). In contrast to CA 15-3, the CEA decrease alone, independent from the level of the pre-operative value, was a strong predictor for early relapse and death of breast cancer (Figure 3).
In multivariate analysis, independent prognostic factors for DFS in 658 patients with 160 recurrences were tumour size, lymph node status, hormone receptors and decrease of the pre-operative CEA value ⩽33% (Table 2). It is notable that 1 year after primary therapy the hazard ratio for relapse for both decrease of CEA values and progesterone receptor diminished compared with the initial risk. Tumour grade, CA 15-3 and pre-operative values of CEA were without significance for relapse.
In 720 patients with 74 tumour associated deaths, tumour size, lymph node status, hormone receptor status and the decrease of CEA were independent predictors for death from disease in multivariate analysis (Table 2). In contrast to relapse-free survival, only the hazard ratio for the hormone receptor status was influenced by time, the decrease of CEA level showed no time dependency. Likewise as for DFS, grading, CA 15-3 and the pre-operative values of CEA were without influence on death from disease.
Discussion
CEA and CA 15-3 are the most thoroughly investigated serum tumour markers in breast cancer. It is generally agreed that tumour markers in breast cancer patients are not a tool for primary diagnosis, because of their low sensitivity and specificity (Tondini et al, 1989; Fateh-Moghadam and Stieber, 1993; Lamerz et al, 1993). Their use for early detection of metastases seems to be promising (Lamerz et al, 1991; Stieber et al, 1992; Vizcarra et al, 1994; O'Hanlon et al, 1995) and their use for measuring therapeutic response in metastatic disease is widely accepted (Tondini et al, 1988; Dnistrian et al, 1991; Robertson et al, 1991; Safi et al, 1991). Many studies tried to assess the prognostic role of these tumour markers (some analysed in serum, some in tissue), but most of them had low patient numbers or short follow-up periods, and used only univariate analyses (Myers et al, 1978; Tormey and Waalkes, 1978; Bezwoda et al, 1981; Mansour et al, 1983; Kallioniemi et al, 1988; Hammer et al, 1992; O'Hanlon et al, 1995). To our knowledge, there is no multivariate analysis on CEA in serum, only a few studies performed multivariate analyses on CEA in breast cancer tissue (Esteban et al, 1994; Sundblad et al, 1996). Among the two multivariate analyses of CA 15-3 in serum at the time of diagnosis of breast cancer (Gion et al, 1991; Shering et al, 1998), only Shering had a sufficiently high number of patients. To date, no study has tested both tumour markers CEA and CA 15-3 for independent prognostic value at the time of primary intervention in breast cancer patients.
In the present study, we investigated the association of the serum levels of the tumour markers CEA and CA 15-3 with DFS and DFD in women with breast cancer without metastases at the time of primary diagnosis, in relation to age and the established prognostic factors tumour size, lymph node status, histological grading and hormone receptor status. In accordance to other studies, we found tumour size, lymph nodes, histological grading and hormone receptors to be prognostically significant for DFS and DFD (Chevallier et al, 1988; Carter et al, 1989; Nomura et al, 1992; Carriaga and Henson, 1995).
In most patients, tumour marker levels were found to be very low (Figures 1 and 2), but were higher than average levels in healthy women (CEA 1.0 ng ml−1; CA 15-3 13.6 U ml−1) (Stieber, 1996). We defined the marker cut-off values in our study by the 95 %-percentile of healthy individuals (2.0 ng ml−1 for CEA and 25 U ml−1 for CA 15-3).
We found that elevated pre-operative serum marker values were correlated with early relapse (CA 15-3; P=0.0003) and death from disease (CEA, CA 15-3; P=0.0001 both) in univariate analyses. Possibly, the release of tumour associated antigens at the time of diagnosis proves blood supply respectively vascularisation of the tumour and by consequence the possibility of already existing micrometastases and bad prognosis from the beginning (Gasparini et al, 1997).
By comparing pre- and post-operative values we found a decline in values post-surgery. The post-operative marker values we determined were well comparable to the median of healthy individuals which could be expected as we investigated only patients with complete resection of the tumour (R0 resection). Therefore it is understandable that we found no significant correlation with the recurrence of disease assessing isolated post-operative marker values of CEA and CA 15-3. Other studies that found an association between elevated post-operative levels of CEA and recurrence of disease had only small patient numbers, short follow up and partly investigated advanced stages of disease (Myers et al, 1978; Hammer et al, 1992). This is one of the first studies to relate changes in serum marker levels following surgery to patient outcome.
In those patients where marker levels of CEA decreased more than 33%, a significantly higher risk for relapse and death from disease (both P=0.0001) in univariate analyses was observed. The general opinion that R0-resection corresponds to good prognosis is not in contradiction to our findings, because we only observed patients with complete resection of the tumour.
It is surprising that we found the high prognostic relevance of the decrease only for CEA, whereas for CA 15-3 a significant worse prognosis can be seen only for patients with high pre-operative tumour marker values and significant decrease after surgery (Figures 2 and 3). This could probably be due to the fact that up to now the reproducibility of CA 15-3 values at low concentrations is unsatisfactory.
In multivariate analysis, this decrease of CEA proved to be an independent prognostic factor (Table 2). The results for CA 15-3 were comparable to CEA in univariate analyses but showed no significance in multivariate analysis at least when both markers were included in the model simultaneously. This could be due to the fact that the correlation with tumour size is higher for CA 15-3 than for CEA.
As we determined both markers, CEA and CA 15-3, it is difficult to compare our study with the two other relevant studies of Gion and Shering (Gion et al, 1991; Shering et al, 1998). Although Gion found, in accordance to us, that pre-operative serum levels of CA 15-3 had no prognostic relevance in multivariate analysis, the number of patients and the unknown number of relapse and death from disease is probably too small to give reliable results.
In contrast Shering et al (1998) reported on a high number of patients and found a prognostic relevance for pre-operative CA 15-3 values. The prognostic relevance of a single factor in multivariate analysis depends on which and how other factors are included in the model. Thus, our results would be similar to those of Shering et al (1998), if CEA was not included in the analysis.
Conclusion
In this study the post-operative decrease of the serum tumour marker CEA was a strong independent prognostic factor for disease free survival and death from disease in breast cancer patients. To our knowledge, this is the first study reporting on the high prognostic relevance of the decline of tumour associated antigens. It is also the largest study to-date to have analysed the prognostic value of serum tumour markers in breast cancer. An advantage of our approach for clinical practice would be the independence from tumour tissue.
In addition to those prognostic factors being already indicative for adjuvant therapy like lymph nodes and grading, a randomized prospective therapy intervention study based on the decrease of CEA would be needed to prove the relevance of our findings.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bezwoda W, Derman D, Bothwell T, MacPhil P, Levin J, DeMoor N (1981) Significance of serum concentrations of carcinoembryonic antigen, ferritin, and calcitonin in breast cancer. Cancer 48: 1623–1628
Carriaga MT, Henson DE (1995) The histologic grading of cancer. Cancer 75: 406–421
Carter CL, Allen C, Henson DE (1989) Relationship of tumorsize, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63: 181–187
Chevallier B, Heintzmann F, Mosseri V, Dauce JP, Bastit P, Graic Y, Brunelle P, Basuyau JP, Comoz M, Asselain B (1988) Prognostic value of estrogen and progesterone receptors in operable breast cancer. Results of a univariate and multivariate analysis. Cancer 62: 2517–2524
Clark GM, Sledge Jr GW, Osborne CK, McGuire WL (1987) Survival from first recurrence: Relative importance of prognostic factors in 1.015 breast cancer patients. J Clin Oncol 5: 55–61
Cox DR (1972) Regression models and life-tables. J R Stat Soc 34: 187–200
Dnistrian AM, Schwartz MK, Greenberg EJ, Smith CA, Schwartz DC (1991) CA 15-3 and carcinoembryonic antigen in the clinical evaluation of breast cancer. Clinica Chimica Acta 200: 81–94
Esteban JM, Felder B, Ahn C, Simpson JF, Battifora H, Shively JE (1994) Prognostic relevance of carcinoembryonic antigen and estrogen receptor status in breast cancer patients. Cancer 74: 1575–1583
Fateh-Moghadam A, Stieber P (1993) Sensible use of tumour markers 2nd edn Marloffstein-Rathsberg: Hartmann-Verlag
Gasparini G, Toi M, Gion M, Verderio P, Dittadi R, Hanatani M, Matsubara I, Vinante O, Bonoldi E, Boracchi P, Gatti C, Suzuki H, Tominaga T (1997) Prognostic significance of vascular endothelial growth factor protein in node-negative breast carcinoma. J Natl Cancer Inst 89: 139–147
Gion M, Mione R, Nascimben O, Valsecchi M, Gatti C, Leon A, Bruscagnin G (1991) The tumour associated antigen CA 15.3 in primary breast cancer. Evaluation in 667 cases. Br J Cancer 63: 809–813
Hammer J, Track C, Hohenwallner W, Seewald DH, Zoidl JP, Wimmer E (1992) MCA and CA 15-3 in der Verlaufskontrolle bei Patientinnen mit Mammakarzinom. Strahlenther Onkol 168: 102–106
Kallioniemi OP, Oksa H, Aaran RK, Hietanen T, Lehtinen M, Koivula T (1988) Serum CA 15-3 assay in the diagnosis and follow-up of breast cancer. Br. J. Cancer 58: 213–215
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481
Lamerz R, Reithmeier A, Stieber P, Eiermann W, Fateh-Moghadam A (1991) Role of Blood Markers in the Detection of Metasases from Primary Breast Cancer. Diagn Oncol 1: 88–97
Lamerz R, Stieber P, Fateh-Moghadam A (1993) Serum marker combinations in human breast cancer (review). In Vivo. 7: 607–614
Mansour EG, Hastert M, Park CH, Koehler KA, Petrelli M (1983) Tissue and plasma carcinoembryonic antigen in early breast cancer: a prognostic factor. Cancer 51: 1243–1248
Mansour EG, Ravdin PM, Dressler L (1994) Prognostic factors in early breast carcinoma. Cancer 74: 381–400
McGuire WL, Clark GM (1992) Prognostic factors and treatment decisions in axillary-node-negative breast cancer. N Engl J Med 326: 1756–1761
Myers RE, Sutherland DJ, Meaking JW, Kellen JA, Malkin DG, Malkin A (1978) Carcinoembryonic Antigen in Breast Cancer. Cancer 42: 1520–1526
Nomura Y, Miura S, Koyama H, Enomoto K, Kasumi F, Yamamoto H, Kimura M, Tominaga T, Iino H, Morimoto T (1992) Relative effect of steroid hormone receptors on the prognosis of patients with operable breast cancer: a univariate and multivariate analysis of 3089 Japanese patients with breast cancer from the study group of the Japanese Breast Cancer Society on hormone receptors and prognosis in breast cancer. Cancer 69: 153–164
O'Hanlon DM, Kerin MJ, Kent PJ, Skehill R, Maher D, Grimes H, Given HF (1995) A prospective evaluation of CA 15-3 in Stage I carcinoma of the breast. J Am Coll Surg 180: 210–212
Robertson JFR, Pearson D, Price MR, Selby C, Blamey RW, Howell A (1991) Objective measurement of therapeutic response in breast cancer using tumor markers. Br J Cancer 64: 757–763
Safi F, Kohler I, Röttinger E, Beger HE (1991) The value of the tumor marker CA 15-3 in diagnosing and monitoring breast cancer. Cancer 68: 574–582
Schmitt M, Thomssen C, Ulm K, Seiderer A, Harbeck N, Höfler H, Jänicke F, Graeff H (1997) Time-varying prognostic impact of tumor biological factors urokinase (uPA), PAI1 and steroide hormone receptor status in primary breast cancer. Br J Cancer 76: 306–311
Shering SG, Sherry F, McDermott EW, O'Higgins NJ, Duffy MJ (1998) Preoperative CA 15-3 concentrations predict outcome of patients with breast carcinoma. Cancer 83: 2521–2527
Stieber P, Nagel D, Ritzke C, Rössler N, Kirsch CM, Eiermann W, Fateh-Moghadam A (1992) Significance of bone alkaline phosphatase, CA 15-3 and CEA in the detection of bone metastases during the follow-up of patients suffering from breast carcinoma. Eur J Clin Chem Clin Biochem 30: 12 809–814
Stieber P (1996) Möglicher Einsatz der Tumormarker in der Nachsorge. Der Bay Int 16: 1 42–54
Sundblad AS, Pellicer EM, Ricci L (1996) Carcinoembryonic antigen expression in Stages I and II breast cancer: its relationship with clinicopathologic factors. Hum Pathol 27: 297–301
Tondini C, Hayes DF, Gelman R, Henderson IC, Kufe DW (1988) Comparison of CA 15-3 and carcinoembrionic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res 48: 4107–4112
Tondini C, Hayes DF, Kufe DW (1989) Circulating tumor markers in breast cancer. Hematol/Oncol Clin North Am 3: 4 653–674
Tormey D, Waalkes T (1978) Clinical correlation between CEA and breast cancer. Cancer 42: 1507–1511
Vizcarra E, Lluch A, Cibrian R, Jarque F, Garcia Conde J (1994) CA 15.3, CEA and TPA tumor markers in the early diagnosis of breast cancer relapse. Oncology 51: 6 491–496
Acknowledgements
Parts of this publication are from the doctoral thesis of Friedrich G Ebeling, at the Ludwig-Maximilians-University Munich.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ebeling, F., Stieber, P., Untch, M. et al. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br J Cancer 86, 1217–1222 (2002). https://doi.org/10.1038/sj.bjc.6600248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6600248
Keywords
This article is cited by
-
MicroRNA-21 expression, serum tumor markers, and immunohistochemistry in canine mammary tumors
Veterinary Research Communications (2022)
-
Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer
Nature Communications (2021)
-
An electrochemical immunosensor for the detection of carcinoembryonic antigen based on Au/g-C3N4 NSs-modified electrode and CuCo/CNC as signal tag
Microchimica Acta (2021)
-
Bimetallic PtCu nanoparticles supported on molybdenum disulfide–functionalized graphitic carbon nitride for the detection of carcinoembryonic antigen
Microchimica Acta (2020)
-
Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study
Breast Cancer (2020)