Abstract
Design
This was a randomised controlled trial (RCT) of the treatment of oral leukoplakia with the carotenoid lycopene.
Intervention
A total of 58 patients received either 8 mg oral lycopene in two doses daily (n=20), 4 mg oral lycopene in two doses daily (n=18) or placebo capsules (n=18), for a 3-month period. Progress of patients was followed for a further 2 months.
Outcome measures
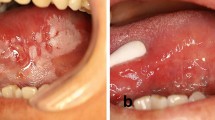
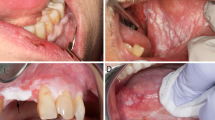
An objective clinical response, evaluated by bidimensional measurement of the lesion and colour photography, was classified as complete, partial, stable or progression. Histological status was categorised and ranked as normal (0), atypical hyperplasia (1), mild dysplasia (2), moderate dysplasia (3) or severe dysplasia (4). Histological response was then described by the change in rank, for example, from moderate dysplasia (3) to atypical hyperplasia (1) would indicate an improvement of 2 units.
Results
There was no significant difference in the clinical response of people who took 8 mg lycopene compared with those taking 4 mg lycopene. The clinical responses measured in both these groups were significantly greater, however, than those in the control group (P<0.01). The response, assessed histologically, after the 8-mg lycopene treatment was significantly better than that from 4 mg lycopene (P<0.05) and than the response seen in the control group (P<0.001). Patients taking 4 mg lycopene also responded significantly better than those in the control group (P<0.05).
Conclusions
Oral lycopene appears, from this small RCT conducted over 5 months, to be effective in the treatment and management of oral leukoplakia.
Similar content being viewed by others
Commentary
Oral leukoplakia is a diagnosis given to a white patch that cannot be categorised. Once a histological diagnosis is made, it is useful to refer to it either by causal factor, for example, candidal leukoplakia, or by degree of dysplasia.1 An international meeting clarifying these definitions reported its findings and also suggested a method of staging these lesions.1 This has been further commented upon by Van der Waal and Axell2 and Schepman and van der Waal3 and it is a pity that this study did not adopt this methodology. This staging not only includes the different forms of dysplasia but also takes into account the size of the lesion.
A recent Cochrane systematic review on treatment of leukoplakia also provides some guidelines for future RCT in this field.4 In their paper, Lodi et al5 point out that no RCT conducted to date exceeds 15 months and yet there is evidence to show that malignant transformation increases with duration of follow-up.6 They also point out that many researchers use outcomes other than malignant transformation, for example, histological diagnosis or resolution of lesion. There are problems with using these outcomes because there is little evidence for their predictive value and it has been shown that outcomes such as dysplasia are subject to high observer variation.7, 8 Many journals have now adopted the CONSORT guidelines for reporting of RCT: this study would have been easier to follow if it had used this format.
There are many omissions in the methodology that affect the reader's ability to interpret the study. The following data are missing: how the trial population was selected; how many people did not consent to the trial; how well-matched the groups were in terms of dysplasia (the control group had no severe dysplastic lesions); what the tobacco use of the population was; what method of randomisation was used; how clinicians and patients were blinded; how many pathologists were involved; how many subjects had biopsies taken pre- and post-treatment; how the site of biopsy was determined, especially if the lesion had disappeared; how the power of the study was determined; how complete the follow-up was; and, finally, how many patients did not complete the full protocol.
The authors point out that many of these lesions are related to smoking or chewing tobacco and yet no details of these habits are provided, either at onset of the study or at its conclusion. Gupta et al9 showed how lesions can disappear after merely cessation of tobacco use. The groups should have been compared using this parameter and the level of dysplasia.
The results also do not specify at which timepoint they were taken, whether at the end of the active use of the drug or at the end of the follow-up period. If the latter, were there any regressions? Data about side effects and toxicity are only provided in the discussion and these are said to be non-existent.
Owing to these problems, it is impossible to draw any firm conclusions from this study in the form in which it is currently reported. It seems feasible to carry out further RCT with lycopene, however, using the criteria suggested by Lodi et al,5 the staging system and CONSORT guidelines.
References
Axell T, Pindborg JJ, Smith CJ, van der Waal I . Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18–21 1994. International Collaborative Group on Oral White Lesions. J Oral Pathol Med 1996; 25:49–54.
van der Waal I, Axell T . Oral leukoplakia: a proposal for uniform reporting. Oral Oncol 2002; 38:521–526.
Schepman KP, van der Waal I . A proposal for a classification and staging system for oral leukoplakia: a preliminary study. Eur J Cancer B Oral Oncol 1995; 31B:396–398.
Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A . Interventions for treating oral leukoplakia (Cochrane Review). In: The Cochrane Library Issue 3, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A . Systematic review of randomized trials for the treatment of oral leukoplakia. J Dent Educ 2002; 66:896–902.
Shiu MN, Chen TH, Chang SH, Hahn LJ . Risk factors for leukoplakia and malignant transformation to oral carcinoma: a leukoplakia cohort in Taiwan. Br J Cancer 2000; 82:1871–1874.
Abbey LM, Kaugars GE, Gunsolley JC, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 80:188–191.
Karabulut A, Reibel J, Therkildsen MH, Praetorius F, Nielsen HW, Dabelsteen E . Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med 1995; 24:198–200.
Gupta PC, Mehta FS, Pindborg JJ, et al. Primary prevention trial of oral cancer in India: a 10-year follow-up study. J Oral Pathol Med 1992; 21:433–439.
Author information
Authors and Affiliations
Additional information
Address for correspondence: Mohitpal Singh, Department of Oral Medicine and Radiology, Pacific Dental College and Hospital, Debari, Udaipur 313024, India. E-mail: mohitpalsingh@yahoo.com.
Singh M, Krishanappa R, Bagewadi A, Keluskar V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncol 2004; 40:591–596.
Rights and permissions
About this article
Cite this article
Zakrzewska, J. Oral lycopene — an efficacious treatment for oral leukoplakia?. Evid Based Dent 6, 17–18 (2005). https://doi.org/10.1038/sj.ebd.6400285
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ebd.6400285