Abstract
Neocentromeres are rare functional centromeres formed within noncentromeric chromosomal regions. We report the finding of a neocentromere in a very rare class II analphoid chromosome. This neocentromere was detected prenatally in a fetus with the karyotype: 47,XY,del(4)(p15.3q21.1),+r(4)(p15.3q21.1).ish del(4)(D4S3360+,WHS+,D4Z1−,4qsubtel+),r(4)(D4S3360−,WHS−,D4Z1+,4qsubtel−)de novo. The fetus was missing one normal chromosome 4 but had a ring chromosome, consisting of the pericentromeric region of chromosome 4, and a deleted chromosome 4, the reciprocal product of the ring formation. In situ hybridization established that the chromosome 4 pericentromeric heterochromatin was located on the ring chromosome, while the Wolf-Hirschhorn critical region and chromosome 4 subtelomeric regions were present on the deleted chromosome. A C-band-negative constriction was observed in band 4q21.2 of the deleted chromosome 4, indicating that a neocentromere had been formed in this band, allowing stable segregation during cell division. This chromosome abnormality was detected in cultured amniocytes from a 20-week pregnancy presenting with intrauterine growth retardation and echogenic bowel. The pregnancy resulted in intrauterine death at 33–34 weeks. Despite the apparently balanced karyotype, the fetus is likely to have been phenotypically impaired due to disruption of genes by the neocentromere, rearrangement and ring chromosome formation. There has been one previous report of neocentromere formation in band 4q21; the observation presented here might refine a putative common neocentromeric site to sub-band 4q21.2.
Similar content being viewed by others
Introduction
Centromeres are specialized chromosomal structures that function to ensure correct segregation of chromosomes and chromatids during meiosis and mitosis. Centromeres characteristically contain an abundance of α-satellite DNA-tandem repeats of an AT-rich, 171-base-pair unit, forming up to 3–4 megabases on each chromosome. While the introduction of α-satellite DNA can promote de novo centromere formation,1 the formation of neocentromeres and the inactivation of endogenous centromeres in dicentric chromosomes suggest that certain reversible epigenetic modifications also confer centromeric function.2
Neocentromeres are functional centromeres formed from previously noncentromeric chromosomal regions. Although one neocentromere has been reported in cis with a canonical centromere,3 they are usually formed when a chromosome rearrangement separates a chromosomal fragment from its endogenous centromere, thus preserving the acentric fragment from loss during cell division. The mitotic stability of neocentromeres is well documented.4 Moreover, recent cases have reported the inheritance of ‘neocentromeric’ chromosomes suggesting that they also have the capacity to function successfully during meiosis.3,5
Neocentromeres are usually devoid of α-satellite DNA. However, studies to date suggest that they share the structural and behavioral characteristics of canonical centromeres; they associate with many functionally important centromere-specific proteins such as CENP-A and heterochromatin proteins (eg hHP1β),1 they form normal end-on associations with kinetochores that have normal morphology and are the same size as kinetochores of other chromosomes of a comparable size5 and replication timing is delayed to the third quarter of S phase (in line with other centromeres), once a neocentromere is activated.6
Preliminary analysis of the DNA sequence across neocentromeric regions suggests the presence of features that may predispose these sites to neocentromere formation: they have a high AT content (∼65%, comparable with the AT content of α-satellite DNA), a high content of other repeat elements6,7 and fewer SINES and potential genes than the surrounding region.6 Furthermore, comparative analysis of primate α-satellite DNA with a human neocentromere revealed sequence symmetries and conserved base motifs that indicate shared higher order structural features.8
Neocentromeres present a potentially powerful tool for elucidating complex centromere structure and function. In addition, they have been used to construct human artificial chromosomes, a potential alternative vector system for gene therapy.1 However, naturally occurring neocentromere formation is a rare phenomenon and the majority reported to date have been found on supernumerary chromosomes, formed from terminal chromosome regions.2 We present here a complex chromosome rearrangement with neocentromere formation in proximal 4q, detected prenatally in a fetus with severe intrauterine growth retardation (IUGR).
Materials and methods
Clinical details
MR was a 43-year-old Caucasian lady with a nonconsanguineous partner of Asian origin. She had previously had a complete miscarriage at 11 weeks gestation. She did not drink or smoke and there was no relevant past medical history. In this pregnancy, the first trimester scan showed uterine fibroids, but normal fetal growth and no obvious fetal abnormalities. A nuchal translucency scan gave a trisomy risk of one in 198.
Routine anomaly scan at 20 weeks showed an echogenic bowel, short femurs and mild ventricular dilatation. Parental cystic fibrosis carrier tests were negative. Amniocentesis was performed and demonstrated an abnormal male karyotype. Both parents had a normal karyotype.
Genetic counselling was difficult because of uncertainty as to the effect of the ring chromosome and neocentromere. The patient was counselled that the IUGR was likely to be related to the chromosomal rearrangement and that there was a significant risk of learning disability. Although the long-term outcome was uncertain, she opted to continue the pregnancy and have regular ultrasound scans.
At 25 weeks, scanning demonstrated that growth was symmetrically well below the fifth centile. A fetal cardiology scan was normal. One more scan at 30 weeks showed that the fetus was continuing to grow along the same centile curve and an umbilical artery Doppler detected absent end diastolic flow.
At 32 weeks, oligohydramnios was noted. No fetal movements were felt after 33 weeks and intrauterine death was confirmed at 34 weeks.
On delivery, the baby weighed 640 g (<0.4th centile) and was macerated. Anal atresia and neck webbing were evident on clinical examination. The umbilical cord had two vessels. A post mortem was not performed. There was no evidence of chorioamnionitis and the placenta had a small infarct (involving less than 5% of the placental parenchyma).
Cytogenetics
Metaphase spreads were prepared from two monolayer cultures of amniocytes from the fetus and peripheral blood lymphocytes from the parents by standard procedures. GTG-banding on all samples and C-banding on fetal metaphase spreads were performed according to standard protocols.
Molecular cytogenetics
Fluorescence in situ hybridization (FISH) studies on metaphase spreads of cultured fetal amniocytes were carried out according to the manufacturer's instructions. Probe specific for locus D4Z1 (α-satellite DNA) at the chromosome 4 centromere (Vysis Inc., Downers Grove, IL, USA), subtelomeric probes specific for the short and long arms of chromosome 4 (D4S3360 and 4qsubtel, respectively) (Qbiogene, Illkirch, France) and probe specific for the Wolf-Hirschhorn critical region (WHS) (Vysis Inc., Downers Grove, IL, USA) were applied.
Results
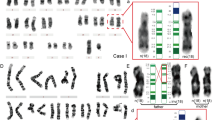
G-banded chromosome analysis revealed an apparently balanced rearrangement of chromosome 4 (Figure 1). The ISCN9 karyotype was: 47,XY,del(4)(p15.3q21.1),+r(4)(p15.3q21.1),ish del(4)(D4S3360+,WHS+,D4Z1,−4qsubtel+),r(4)(D4S3360−,WHS−,D4Z1+,4qsubtel−)de novo. The fetus was missing one normal chromosome 4 but had a ring chromosome, consisting of the pericentromeric region of chromosome 4, and a deleted chromosome 4, formed by fusion of the distal acentric fragments. A constriction was observed in sub-band 4q21.2 of the deleted chromosome 4, indicating that a neocentromere had been formed in this sub-band. The ring chromosome was lost in 4/100 metaphases examined.
(a) Partial G-banded metaphase. The long arrow denotes the normal chromosome 4, the short arrow denotes the deleted chromosome 4 and the round-headed arrow denotes the ring chromosome. (b) Computer-generated idiogram. Black arrows indicate the deletion breakpoints; the red arrow denotes the position of the neocentromere.
C-banding (Figure 2a) and FISH with chromosome 4 centromere specific probe (Figure 2b) showed that the ring chromosome contained the chromosome 4 centromere and that no α-satellite DNA or C-band-positive material was present on the acentric chromosome, consistent with neocentromere formation.
Partial metaphases of: (a) C-banding, (b) FISH using probes specific for the chromosome 4 centromere (D4Z1; green signals) and Wolf-Hirschhorn critical region (WHS; red signals) and (c) FISH using subtelomeric probes specific for the chromosome 4 short arm (D4S3360; green signals) and long arm (4qsubtel; red signals).
Subtelomeric probes for the short and long arms of chromosome 4 and the Wolf-Hirschhorn critical region all hybridized, as expected, to the neocentric chromosome (Figure 2c).
Discussion
Approximately 60 human neocentromeres, on derivatives of 18 different chromosomes, have been described to date in the literature. The majority have been present in supernumerary chromosomes formed from inversion duplications of terminal chromosomal regions, resulting in tetrasomy or trisomy for the duplicated region.10 These have been termed class I type neocentromere analphoid chromosomes.4 Only nine neocentromeres have been documented on acentric fragments derived from interstitial deletion events (termed class II type analphoid chromosomes). The deletion is followed by fusion of the two more distal chromosome fragments and thus theoretically results in a balanced karyotype. A summary of reported chromosome rearrangements with class II analphoid chromosomes is provided in Table 1.
The current case is the second report of neocentromere formation in chromosome band 4q21. A class II neocentromere containing ring chromosome, formed from band 4q21 was previously reported in a boy with hyper-IgE syndrome and autism.11 The recurrence of a neocentromere in band 4q21 suggests that this chromosome band contains a site predisposed to neocentromere formation. The present case therefore may refine a putative common neocentromeric site to sub-band 4q21.2.
The most frequently recurring neocentromeres reported to date occur in chromosome bands 13q21 and 13q32 and the distal regions of 3q and 15q.10 It is currently unclear to what extent this situation reflects the frequency of particular chromosome rearrangements giving rise to acentric fragments, the viability of aneuploidy resulting from the rearrangement or reporting bias. It has been suggested that palindromic sequences formed by U-type exchange during the production of inverted duplications may direct epigenetic factors and promote neocentromere formation.12
In common with the current case, a ring chromosome was formed by the deleted segment in 7/9 of previously reported chromosome rearrangements with class II neocentromere containing chromosomes.5,11,13,14,15,16,17,18,19 In the majority of cases, the ring chromosome was lost in a small proportion of cells irrespective of whether the ring contained an endogenous centromere or a neocentromere, probably due to the instability of the ring structure during cell division. The even distribution of neocentromere formation between rod and ring structures and the observation that neocentromere formation does not usually occur at or near deletion breakpoints suggest that neocentromere formation is not promoted by ring chromosome formation. In two of the previously reported ‘class II’ cases, the deleted segments remained as linear structures. One of these fragments had undergone telomere capture and, as with the ring chromosomes, was also lost in only a small proportion of cells (10%),5 while the other did not demonstrate telomeric sequences and was more frequently lost (62%)17 suggesting that ring formation or telomere capture is necessary for stabilization of the deleted segment.
The low levels of ring chromosome loss observed in the amniocytes of the present case and in other cases of class II analphoid markers suggests that there is a strong selective advantage for cells with a balanced karyotype. The initial ultrasound finding of IUGR and no gross structural abnormality in the current case was consistent with ‘ring syndrome’ (mild–moderate learning difficulties and growth retardation regardless of the genetic make-up of the ring).20
Loss of the ring chromosome would result in a cell line monosomic for the chromosomal segment 4p15.3–4q21.2. There have been no previously published cases of monosomy for this region; however, smaller interstitial deletions (4q12–21) within the putatively monosomic segment have been reported with low birth weight and failure to thrive.21 Furthermore, one report of prenatal detection of a deletion of 4q12–21.1 described a fetus phenotypically very similar to the current case (it was compatible with menstrual dating except for shortening of femur length on ultrasound at 20 weeks gestation, showed IUGR and a cardiac abnormality at 26 weeks, and at 28 weeks all growth measurements were below the 10th percentile).22 The phenotypic similarity suggests that loss of the ring chromosome is significant. Alternatively, disruption of a gene at the shared chromosome breakpoint at 4q21.1 may account for the phenotypic resemblance.
Further phenotypic abnormality may have been caused by transcriptional silencing of genes around the neocentromeric site. Transcriptional silencing of genes flanking pericentromeric heterochromatin and of euchromatic genes mislocated to heterochromatin has been documented in a number of species,1 while proteins that usually localize to pericentromeric heterochromatin have been observed to associate with neocentromeres in the regions flanking the CENP-A binding domain.1 However, the comparatively mild phenotype of the patient reported by Grimbacher et al.11 suggests that neocentromerization in band 4q21 is unlikely to be the sole reason for the severe phenotype in this case, assuming that the neocentromere in both cases has formed at a common site. Nevertheless, disruption of genes due to neocentromerization, the deletion breakpoints or position effects on the rearranged chromosome 4 may also have contributed to the severe phenotype.
Given their mitotic stability, the high frequency of mosaicism observed among class I analphoid markers has led to the suggestion that neocentromerization does not occur synchronously with the formation of the supernumerary chromosome fragment at meiosis, but takes place at an early postzygotic cell cycle.4 The possibility of disruption of fetal development due to an early cell line monosomic for most of chromosome 4, subsequently selected against and lost, cannot therefore be excluded.
This is the second report of neocentromere formation in band 4q21 and thus refines a putative neocentromeric site to sub-band 4q21.2. Such reports are of value in the elucidation of factors influencing neocentromere formation.
References
Choo KH : Domain organization at the centromere and neocentromere. Dev Cell 2001; 1: 165–177.
Warburton PE, Dolled M, Mahmood R et al: Molecular cytogenetic analysis of eight inversion duplications of human chromosome 13q that each contain a neocentromere. Am J Hum Genet 2000; 66: 1794–1806.
Tyler-Smith C, Gimelli G, Giglio S et al: Transmission of a fully functional human neocentromere through three generations. Am J Hum Genet 1999; 64: 1440–1444.
Voullaire L, Saffery R, Earle E et al: Mosaic inv dup (8p) marker chromosome with stable neocentromere suggests neocentromerization is a post-zygotic event. Am J Med Genet 2001; 102: 86–94.
Wandall A, Tranebjaerg L, Tommerup N : A neocentromere on human chromosome 3 without detectable alpha-satellite DNA forms morphologically normal kinetochores. Chromosoma 1998; 107: 359–365.
Lo AW, Craig JM, Saffery R et al: A 330 kb CENP-A binding domain and altered replication timing at a human neocentromere. EMBO J 2001; 20: 2087–2096.
Satinover DL, Vance GH, Van Dyke DL, Schwartz S : Cytogenetic analysis and construction of a BAC contig across a common neocentromeric region from 9p. Chromosoma 2001; 110: 275–283.
Koch J : Neocentromeres and alpha satellite: a proposed structural code for functional human centromere DNA. Hum Mol Genet 2000; 9: 149–154.
Mitelman F (ed) An international system for human cytogenetic nomenclature (ISCN). Basel: S Karger.
Amor DJ, Choo KH : Neocentromeres: role in human disease, evolution, and centromere study. Am J Hum Genet 2002; 71: 695–714.
Grimbacher B, Dutra AS, Holland SM et al: Analphoid marker chromosome in a patient with hyper-IgE syndrome, autism, and mild mental retardation. Genet Med 1999; 1: 213–218.
Reddy KS, Sulcova V, Schwartz S et al: Mosaic tetrasomy 8q: inverted duplication of 8q23.3qter in an analphoid marker. Am J Med Genet 2000; 92: 69–76.
Maraschio P, Tupler R, Rossi E et al: A novel mechanism for the origin of supernumerary marker chromosomes. Hum Genet. 1996; 97: 382–386.
Petit P, Fryns JP : Interstitial deletion 2p accompanied by marker chromosome formation of the deleted segment resulting in a stable acentric marker chromosome. Genet Couns 1997; 8: 341–343.
Voullaire LE, Slater HR, Petrovic V, Choo KH : A functional marker centromere with no detectable alpha-satellite, satellite III, or CENP-B protein: activation of a latent centromere? Am J Hum Genet 1993; 52: 1153–1163.
Slater HR, Nouri S, Earle E, Lo AW, Hale LG, Choo KH : Neocentromere formation in a stable ring 1p32–p36.1 chromosome. J Med Genet 1999; 36: 914–918.
Depinet TW, Zackowski JL, Earnshaw WC et al: Characterization of neo-centromeres in marker chromosomes lacking detectable alpha-satellite DNA. Hum Mol Genet 1997; 6: 1195–1204.
Knegt AC, Li S, Engelen JJ, Bijlsma EK, Warburton PE : Prenatal diagnosis of a karyotypically normal pregnancy in a mother with a supernumerary neocentric 13q21 → 13q22 chromosome and balancing reciprocal deletion. Prenat Diagn 2003; 23: 215–220.
Higgins RR, W E., Baldinger S, Tschider E, Ahmad J, Schwarz S, Curtis CA : Neocentromere in a ring-shaped chromosome 1. Am J Hum Genet Suppl 2000; 67: A775.
Kosztolanyi G : Does ‘ring syndrome’ exist? An analysis of 207 case reports on patients with a ring autosome. Hum Genet 1987; 75: 174–179.
Strehle EM, Ahmed OA, Hameed M, Russell A : The 4q− syndrome. Genet Couns 2001; 12: 327–339.
Hsu TY, Kung FT, Ou CY et al: Prenatal diagnosis of de novo interstitial deletion of proximal 4q by maternal serum screening for Down syndrome. Prenat Diagn 1998; 18: 1323–1327.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warburton, P., Barwell, J., Splitt, M. et al. Class II neocentromeres: a putative common neocentromere site in band 4q21.2. Eur J Hum Genet 11, 749–753 (2003). https://doi.org/10.1038/sj.ejhg.5201047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201047