Key Points
-
Planimetric methods for the assessment of plaque are more objective than traditional indices.
-
Qualitative light induced fluorescence (QLF) offers a novel and effective planimetric system.
-
The removal of a detergent from a dentifrice does not adversely affect plaque regrowth.
-
The addition of whitening agents does not adversely affect plaque regrowth.
Abstract
Objective To determine the effects of a detergent-free, whitening dentifrice using an in vivo plaque regrowth model with the novel application of QLF as a planimetric analysis tool.
Method A total of 20 subjects took part in a double blind, single-centre, crossover study in which slurry rinses were the only form of plaque control over a 5-day period. Following a washout and prophylaxis the subjects used 2 daily rinses in the absence of all other plaque control methods. Subjects returned to the clinic on the afternoon of day 5 when plaque was disclosed and assessed by the plaque index and area using both a photographic and novel fluorescent planimetric technique.
A further 9-day washout was carried out and the rinse period repeated to ensure that each subject had used both experimental and comparator slurries.
Results Twenty subjects completed the trial. The test product showed a significant inhibition of plaque re-growth (16.9%) compared with a fluoride-matched comparator using the Turesky index (P < 0.0001), the photographic planimetric technique (17.5%) (P < 0.0001) and the novel QLF technique (18.4%) (P < 0.0001).
Conclusion The results confirm that plaque inhibition capability of a detergent-free whitening dentifrice is at least as effective as a fluoride matched comparator. QLF is a promising tool for disclosed plaque quantification.
Similar content being viewed by others
Introduction
Dentifrice
There has been a substantial increase in whitening dentifrice sales in the UK in recent years. This consumer interest is also reflected within the academic community and a substantial number of studies have been conducted looking at the mode of action and efficacy of such products.1,2,3,4,5 The majority of the studies have concentrated upon the ability of the dentifrices to remove an extrinsic stain from teeth, and few have considered the plaque inhibitory effects of pastes with whitening actives added. It is essential that, in addition to any cosmetic benefits such pastes may bring, the dentifrices do not adversely affect fluoride delivery and plaque removal and inhibition.
The addition of foaming agents to dentifrices has been associated with an increased inhibition of plaque.6 However, detergents have been linked to oral ulceration in susceptible individuals,7 and therefore there has been a desire to create a dentifrice without detergents that retains a plaque inhibitory action. Several additional side effects have been attributed to detergents including: hypersensitivity to metal ions, increased mucosal permeability to oil and water soluble compounds, interaction with the deposition of fluoride on dental enamel, and possibly a reduced cariostatic effect.7,8
The dentifrice under investigation within this study (Yotuel, Biocosmetics, Madrid, Spain) is a sodium lauryl sulphate (detergent, SLS) free, low abrasion (RDA)37 dentifrice containing both xylitol and fluoride. The whitening efficacy of the paste has been described in a previous study.9 The purpose of this trial is to investigate the effect of SLS removal and the incorporation of whitening agents on the plaque inhibitory action of the paste using a well-accepted plaque regrowth model.10,11,12
Plaque measurement
There are a multitude of plaque indices available to researchers examining products or therapies, to detect, measure and describe the plaque effects of such interventions. Indices in current use generally estimate plaque quantity by either the area of tooth covered or the thickness of the plaque in the area measured. The indices can be regarded as non-linear and therefore should be treated as scores assigned on an integer scale.13 The plaque index developed by Quigley and Hein14 and modified by Turesky et al.15 is one of the most frequently used indices in product testing. The technique employs disclosed plaque that is counted on the facial and lingual surfaces and emphasises the difference in plaque accumulation in the gingival third.13 The scoring system is as follows:
0 = No plaque
1 = Separate flecks of plaque at the cervical margin of the tooth
2 = A thin continuous band of plaque (up to 1 mm) at the cervical margin
3 = A band of plaque wider than 1 mm but covering less than one third of crown
4 = Plaque covering at least one third but less than two thirds of the crown, and,
5 = Plaque covering two-thirds or more of the crown.
A total score is obtained by dividing the sum of all measurements by the tooth surfaces scored.13 There are however a number of accepted problems with the use of plaque indices. For example using the Quigley Hein index, it would be possible to have a 50% reduction in plaque volume of a single tooth and yet the index score would remain the same, ie by using categorical data there is a loss of resolution, eg there is no 1.5 score. Other difficulties lie with the subjective nature of the indices and the need for examiner training, often increasing the cost of clinical trials, as does the need for a clinician to conduct the examination. The lack of resolution within the indices can require a larger number of participants to take part in a longer running trial in order to statistically separate therapies or products.
To address these issues, many researchers now employ a planimetric system to measure plaque area, and this is usually expressed as percentage plaque index, or PPI, representing the percentage of the tooth surface covered by the disclosed plaque. Many different techniques exist to conduct planimetric analyses but the basic principle is that the tooth is imaged in some way, most often by a photograph, and then computer software is used to detect the plaque and measure the surface area covered. A key principle behind the planimetric techniques is that the components of the image obtained, ie the tooth without plaque, the disclosed plaque and the gingivae can easily be distinguished by the software, ensuring that only the plaque is measured. A number of techniques have been developed to increase the contrast between these elements, for example by using fluorescent dyes.16
The advent of quantitative light-induced fluorescence (QLF) to detect early carious lesions has offered a novel way of obtaining images suitable for planimetric analysis. Under QLF conditions, plaque is visible as an orange area, the teeth are green in colour and the gingivae are black or brown. These elements are very discrete and therefore it is proposed that such images would be highly amenable to planimetric analysis as the image analysis software can easily distinguish between these colours (Fig. 1). Within this trial QLF images will be taken to assess their ability to be used as planimetric images.
Materials and methods
Plaque re-growth study
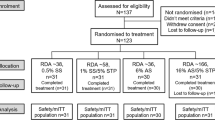
A single-centre, double-blind, single treatment, randomised, controlled, cross-over design was employed for this investigation (Fig. 2). Prior to the start of this study, ethical approval was sought and obtained from the Royal Liverpool and Broadgreen Hospitals Local Ethics Committee, and all volunteers were provided with approved consent forms and subject information sheets. Each consent form was signed and witnessed. The study was conducted in accordance with the Declaration of Helsinki (1964) and subsequent amendments.
Two oral rinses were used in this study — the first, the test product YOTUEL (Biocosmetics, Madrid, Spain) and the second a standard comparator paste. The comparator paste was a fluoride matched paste (1,000 ppm) containing sorbitol, aqua, hydrated silica, glycerine, titanium dioxide, cocamidopropyl betaine, xanthan gum, sodium fluoride, SLS, sodium saccharin, diazolidinyl urea and aroma.
A total of 20 subjects took part, with a mean age of 35 years (±11.7). The volunteers were all dentate with a high standard of oral and gingival health. Exclusion criteria for volunteers included those wearing fixed or removable prostheses, and those with medical or drug histories likely to compromise the trial. Such exclusions included those who had taken antibiotics in the preceding six months, were pregnant, or had used chlorhexidine products within the preceding six weeks.
The sequence of slurry allocation was according to a predetermined randomisation. A washout period of 9 days preceded each rinse phase. During washout, subjects were provided with a standard fluoride toothpaste (Biocosmetics, Spain) and brush (Lactona, The Netherlands) to use in place of their regular oral hygiene products.
At the start of each experimental period (day 1, Monday), subjects attended the dental clinic and were disclosed with PlaqueFinder (Pro-Dentec, Rota-Dent, Cambridge UK) dye, and then provided with a prophylaxis to clean their teeth of plaque and calculus. Re-disclosing, and repeating the prophylaxis as required, confirmed their plaque-free status. Following this, the subjects were provided with their first toothpaste slurry (3 g of product in 10 ml distilled water), and instructed to rinse thoroughly for 1 min. They repeated this on Monday afternoon and twice daily until Friday afternoon (10 rinses in total) and were instructed to refrain from using any other oral hygiene product during this time.
On day 5 (Friday) the subjects returned to the dental clinic. Plaque was again disclosed and assessed on both buccal and lingual surfaces using the Turesky et al.15 modification of the Quigley and Hein plaque index.14 Digital photographs and QLF images were taken of the anterior teeth (canine to canine, Mn, Mx) for planimetric plaque analysis. A headrest assembly was used to ensure that angulations for each of the images was standardised (Fig. 3). Subjects were provided with a further prophylaxis. Each subject then undertook a 9-day washout and presented on Monday for the second phase of the trial.
Planimetric analysis
Planimetric analysis was performed using an image analysis package (Scion Image, Scion Corporation, USA) by a single examiner using the images acquired by the QLF device and the conventional digital photographs. QLF images were available for the maxillary and mandibular anterior teeth on a total of 12 surfaces for analysis. Initially each tooth was selected from the image and subsequently the plaque from that tooth, defined by the orange/red colour removed. Each of these two images (the total tooth image and the plaque only image) was measured at a pixel level. Figure 4 shows an example of this analysis. For the conventional method, the blue-red areas were selected and measured in the same manner. From the pixel measurements the percentage plaque index was calculated and entered into SPSS for later analysis.
Statistical analysis
Whole mouth, mean plaque scores and mean percentage plaque index (PPI) were used for statistical analysis. Statistical assessment showed no skewness in the data, and paired t-test comparisons were deemed appropriate to compare comparator and experimental data. SPSS was used to analyse the data.
Results
Plaque index and area
A full data set was available as all of the 20 subjects successfully completed the study. The mean number of surfaces available for scoring per subject was 54 (±1.9) (not including third molars). No adverse side effects were noted during the duration of the trial. None of the subjects were known or suspected of deviating from the research protocol. Five of the 20 subjects were smokers, with a mean of 14 (±2.3) cigarettes smoked per day. The whole mouth average plaque scores for both the experimental and comparator pastes, and statistical differences between them are listed in Table 1. There was a significant difference in plaque regrowth between the two pastes (P < 0.0001). Numerically the plaque regrowth reduction was 16.09% between the comparator and test paste as measured by the plaque index. A total of 119 surfaces were available for planimetric analysis. Statistical analysis of the conventional and QLF PPI showed a significant reduction in plaque area with the experimental paste compared with the comparator (P < 0.0001), with a 17.15% and 18.21% reduction in plaque re-growth respectively (Table 2).
Discussion
Use of QLF as a planimetric device
As described previously, success in planimetry is based upon a number of factors:17
-
Obtaining high quality and finely focused images
-
Absence of flash artefacts which can confound analysis
-
Standardised size to enable comparisons between visits to be assessed; this could be achieved by either a very small focal distance, or by the use of a scale incorporated into the image
-
The visualisation of the three components of the image: the tooth without plaque, the tooth with plaque, and the surrounding structures (normally gingivae), and
-
Proven reliability and accuracy.
While the above list represents essentials for planimetric imaging, there are a number of additional desirable features:
-
The ability to see a live image, thus enabling immediate review and repeat shot if necessary, not usually available with conventional film photography.
-
The ability to store, archive and retrieve images with ease.
-
The ability to electronically share the images, enabling further examiners to analyse the data.
-
The ability to image posterior teeth without the use of mirrors, including their occlusal surfaces.
-
A short learning curve for the operator acquiring the images and those who analyse them, and
-
Economy.
The QLF device satisfies all of the essentials and all but one of the desirables; it is currently an expensive piece of equipment when compared with simple photographic techniques. However, it is important to note that the device does not require photographic consumables, and therefore operating costs, following initial purchase, are minimal. The QLF devices' lack of specular reflections and its extremely small focal depth ensure that the images are of the highest quality. These physical attributes are combined with a software package (VidRep, Inspektor Research Systems BV, The Netherlands) to make sure that repeated images of the teeth are identical. This is of particular use if you did not want to express a percentage change, but rather, for example, an absolute measurement such as plaque coverage in square millimetres. Indeed, with the power of the Scion Image software, it would be possible to use an actual pixel count, which, in the example given in Figure 4 is a six-figure number and provides a measurement of an extremely high resolution when compared with the somewhat gross indication of plaque coverage provided by the indices.
The separation of the three components of the planimetric image: tooth, plaque and the gingivae is profound with QLF as shown in Figure 1 and Figure 4. The image analysis software can readily detect these distinct areas and the process requires very little subjective input from the operator once the threshold level (the point between which the software considers a pixel plaque or tooth) is set. This threshold is a standardised value for each QLF unit and should be for every trial conducted using that camera and light source. A system of calibration, using a standardized tooth covered with a known area of plaque can be used to ensure that, by thresholding, an image of a tooth by any QLF unit will return the same plaque area. This is particularly useful in multiple-centre trials where several different units may be used and consistency between them, just as consistency between clinical assessors using calibrated Turesky indices, is essential. The QLF does not require any special conditions, beyond those of normal plaque measurements (ie disclosure), to produce these images. This is in contrast to the excellent work by Sagel and colleagues16 who achieved startling separation when using fluorescent light conditions and fluorescein staining of the biofilm (Fig. 5). However, if it is necessary within a trial protocol to use a traditional index as an adjunct, regular disclosing will be required which could be confounded by the fluorescein stain.
a) Actual digital image of the teeth, b) Image analysis software separates the individual elements: uncovered enamel, plaque covered enamel, gingivae, other oral tissues, cheek retractors. Reproduced with kind permission from Karger, Basel. Originally published in Assessment of Oral Health, Diagnostic Techniques and Validation Criteria, Faller R. V. (ed)
A previous study has examined the reliability of the QLF technique, both between and within operators from multiple-centres.18 However, it is important to note that this study was examining the reliability of demineralisation measurement, rather than plaque and while some of the data could reasonably be extrapolated, it is important that further research is conducted within this area to ensure that QLF planimtery is reproducible.
Using the more conventional planimetric method within this trial separated the two dentifrices under investigation. However, the analysis of the images was more complex, with a degree of subjective assessment required on the part of the operator. This was particularly the case when deciding what the threshold should be for the software to discriminate between plaque and gingivae — with both elements having a red appearance. When decided upon, it became apparent that this had to be individually set for each tooth image. This is because of the variation in the photographic appearance of the teeth, as it is difficult to standardise the lighting environment and therefore flash saturation can vary.
One of the limitations of the planimetric methods is that only plaque coverage based upon area is measured — there in no indication of the depth or the density of that plaque.19,20,21 The ability to measure, although indirectly, plaque area within an objective planimetric system would be of advantage to researchers investigating plaque reduction following interventions.22 The QLF technique may offer such measurement opportunities. The intensity of the plaque fluorescence is thought to be related to plaque depth, with the deepest areas normally occurring near the gingival margin. By adjusting the threshold of the image analysis software it is possible to exclude, for example, all but the most fluorescent areas; Figure 6 and Table 3 show the effect of density thresholding on the plaque measurements. Further research is required to link the threshold values within the software (0-255) to actual plaque depth. However, early investigations suggest that the technique may have value. It is important to note that both the traditional index and the planimetric system separated the two pastes, supporting the robustness of both methods. It is the added value of the planimetric technique that supports its use in plaque regrowth trials.
Examples of the effect of thresholding to measure plaque density and depth (see Table 3)
Test product versus comparator
The data suggest that the addition of whitening components and the removal of a detergent (sodium lauryl sulfate, SLS) do not have an adverse influence on the plaque inhibitory effect of the test dentifrice, despite its implicated role in the inhibition of plaque biofilms.23 The absence of the detergent is of interest to those patients who suffer from oral ulceration, particularly of the recurrent aphthous types, as detergents such as SLS added to dentifrices have been shown to exacerbate such conditions.7,8,24 A number of additional side effects have been attributed to SLS including, hypersensitivity to metal ions, increased mucosal permeability to oil and water soluble compounds and an interaction with the deposition of fluoride on dental enamel, possibly reducing the cariostatic effect.23 Further studies are required to substantiate these findings, including a comparison between the Yotuel dentifrice and a true negative control.
While statistically superior to the comparator dentifrice in this trial, when measured by each of the indices, the absolute differences in plaque reduction were small. The results therefore suggest that the absence of a detergent, and the addition of active whitening ingredients do not have an adverse effect on the plaque inhibitory action of the paste. The test product is at least as effective at plaque inhibition as a fluoride matched comparator.
The incorporation of whitening products, and the effect of this on plaque regrowth, will require further investigation using a large number of such pastes to ensure that this cosmetic addition has no deleterious effect on plaque inhibition. Certainly within the current study, the addition of a whitening agent did not adversely affect the paste when compared with a standard, non-whitening paste.
Conclusions
The QLF planimetric method is a promising new technique for plaque measurements within clinical trials and the possibility of simultaneous plaque depth assessment is a novel feature of such objective methods. Further research is required to determine the reliability of the technique between and within examiners in different centres. The detergent free, fluoride and xylitol added whitening toothpaste is effective as a plaque inhibitor when assessed using a regrowth model (Yotuel, Biocosmetics, Madrid, Spain).
References
Sharma NC, Galustians HJ, Qaqish J, et al. The clinical efficacy of Colgate Total Plus Whitening Toothpaste containing a special grade of silica and Colgate Total Toothpaste for controlling breath odor twelve hours after toothbrushing: a single-use clinical study. J Clin Dent 2002; 13: 73–76.
Nathoo S, Petrone ME, DeVizio W, Chaknis P, Volpe AR . A six-week clinical study to compare the stain removal efficacy of three dentifrices. J Clin Dent 2002; 13: 91–94.
Volpe AR, Petrone ME, DeVizio W, Emling RC, Yankell SL . A new perspective on the methodology for clinical efficacy evaluations of tooth whitening dentifrices. J Clin Dent 1999; 10 (3 Spec No): 95–98.
Allen DR, Battista GW, Petrone DM, et al. The clinical efficacy of Colgate Total Plus Whitening Toothpaste containing a special grade of silica and Colgate Total Fresh Stripe Toothpaste in the control of plaque and gingivitis: a six-month clinical study. J Clin Dent 2002; 13: 59–64.
Mankodi S, Sowinski J, Davies R, et al. A six-week clinical efficacy study of a tooth whitening tartar control dentifrice for the removal of extrinsic tooth stain. J Clin Dent 1999; 10: (3 Spec No), 99–102.
Moran J, Addy M, Roberts S . A comparison of natural product, triclosan and chlorhexidine mouthrinses on 4-day plaque regrowth. J Clin Periodontol 1992; 19: 578–582.
Herlofson BB, Barkvoll P . The effect of two toothpaste detergents on the frequency of recurrent aphthous ulcers. Acta Odontol Scand 1996; 54: 150–153.
Herlofson BB, Barkvoll P . Desquamative effect of sodium lauryl sulfate on oral mucosa. A preliminary study. Acta Odontol Scand 1993; 51: 39–43.
Pretty IA, Edgar WM, Higham SM . The use of QLF to quantify in vitro whitening in a product testing model. Br Dent J 2001; 191: 566–569.
Addy M, Renton-Harper P, Newcombe R . Plaque regrowth studies: discriminatory power of plaque index compared to plaque area. J Clin Periodontol 1999; 26: 110–112.
Coessens P, Herrebout F, De Boever JA, Voorspoels J, Remon JP . Plaque-inhibiting effect of bioadhesive mucosal tablets containing chlorhexidine in a 4-day plaque regrowth model. Clin Oral Investig 2002; 6: 217–222.
Sano H, Shibasaki K, Matsukubo T, Takaesu Y . Effect of rinsing with phosphorylated chitosan on four-day plaque regrowth. Bull Tokyo Dent Coll 2001; 42: 251–256.
Fischman SL . Current status of indices of plaque. J Clin Periodontol 1986; 13: 371–374, 379–380.
Quigley G, Hein J . Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc 1962; 65: 26–29.
Turesky S, Gilmore ND, Glickman I . Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol 1970; 41: 41–43.
Sagel PA, Lapujade PG, Miller JM, Sunberg RJ . Objective quantification of plaque using digital image analysis. Monogr Oral Sci 2000; 17: 130–143.
Rekola M, Scheinin A . Quantification of dental plaque through planimetric analysis. Scand J Dent Res 1977; 85: 51–55.
Pretty IA, Edgar WM, Higham SM . QLF quantification of plaque in a re-growth clinical trial. Caries Res 2002; 36: 214–215.
Quirynen M, Dekeyser C, van Steenberghe D . Discriminating power of five plaque indices. J Periodontol 1991; 62: 100–105.
Eaton KA, Kieser JB, Baker R . Assessment of plaque by image analysis. J Clin Periodontol 1985; 12: 135–140.
Soder PO, Jin LJ, Soder B . Computerized planimetric method for clinical plaque measurement. Scand J Dent Res 1993; 101: 21–25.
Loe H . The Gingival Index, the Plaque Index and the Retention Index Systems. J Periodontol 1967; 38: 610–616.
Herlofson BB, Barkvoll P . Oral mucosal desquamation of pre- and post-menopausal women. A comparison of response to sodium lauryl sulphate in toothpastes. J Clin Periodontol 1996; 23: 567–571.
Herlofson BB, Barkvoll P . Sodium lauryl sulfate and recurrent aphthous ulcers. A preliminary study. Acta Odontol Scand 1994; 52: 257–259.
Acknowledgements
The authors would like to thank Dr. Anna Milan for her assistance with the statistics and Dr. Phillip Smith for his suggestions regarding the planimetric system. This study was partially funded by Biocosmetics, Madrid, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Pretty, I., Edgar, W. & Higham, S. A study to assess the efficacy of a new detergent free, whitening dentifrice in vivo using QLF planimetric analysis. Br Dent J 197, 561–566 (2004). https://doi.org/10.1038/sj.bdj.4811809
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4811809
This article is cited by
-
Comparison of Quantitative light-induced fluorescence-digital (QLF-D) images and images of disclosed plaque for planimetric quantification of dental plaque in multibracket appliance patients
Scientific Reports (2020)
-
An in vivo efficacy study of a new detergent free whitening dentifrice
British Dental Journal (2004)