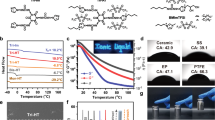
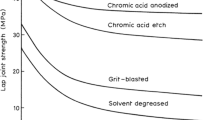
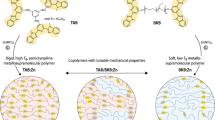
Abstract
The industrial importance of molecular materials chemistry has promoted great interest in areas such as self-assembled surface coatings1, multi-layer formation on solid substrates2, crystallization from solutions3, crystal morphology4 and structure prediction5, solving structures from powders6 and control of polymorphism7. Improvements in our understanding of the role of intermolecular interactions in driving molecular self-assembly and interfacial processes have led to technological advances—both in controlling the assembly of molecules at the nanometre scale, and in manipulating processes and products in which crystal nucleation and growth are key elements8. But there has been relatively little work on molecular-scale engineering at solid–solid interfaces, despite their importance in polymeric composites for structured and electronic applications, in adhesives and in formulated pharmaceutical and agrochemical products. Here we report the use of molecules as tailored adhesives—a molecular ‘glue’ is selected to bond across an interfacial region and hence stabilize a solid–solid interface. We consider a simple interface occurring in a twinned crystal of saccharin; additive molecules with predictable dimensions and hydrogen-bonding functionality can span the interface. The stabilization is reflected in an enhanced frequency of twin-crystal formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Maoz,R., Matlis,S., Dimasi,E., Ocko,B. M. & Sagiv,J. Self-replicating amphiphilic monolayers. Nature 384, 150–153 (1996).
Hatzor,A. et al. Coordination-controlled self-assembled multilayers on gold. J. Am. Chem. Soc. 120, 13469–13477 (1998).
Weissbuch,I., Popvitz-Biro,R., Leiserowitz,L. & Lahav,M. in The Lock and Key Principle (ed. Behr, J. P.) 173–246 (Wiley & Sons, Chichester, 1994).
Docherty,R. & Roberts,K. J. Modelling the morphology of molecular crystals. J. Cryst. Growth 88, 159–168 (1988).
Gavazotti,A. & Fillipini,G. Polymorphic forms of organic crystals at room conditions: thermodynamic and structural implications. J. Am. Chem. Soc. 117, 12299–12305 (1995).
Docherty,R. & Jones,W. in Organic Molecular Solids—Properties and Applications (ed. Jones, W.) Ch. 5 (CRC Press, London, 1997).
Davey,R. J., Blagden,N., Potts,G. D. & Docherty,R. Polymorphism in molecular crystals: stabilization of a metastable form by conformational mimicry. J. Am. Chem. Soc. 119, 1767–1772 (1997).
Davey,R. J., Maginn,S. J. & Polywka,L. in Advances in Industrial Crystallisation 150–165 (Butterworth-Heineman, Oxford, 1991).
Lieberman,H. F., Williams,L., Davey,R. J. & Pritchard,R. G. Molecular configuration at the solid-solid interface: twinning in saccharin crystals. J. Am. Chem. Soc. 120, 686–691 (1998).
Williams-Seton,L., Davey,R. J. & Liebermann,H. F. Solution chemistry and twinning in saccharin crystals: a combined probe for the structure and functionality of the crystal-fluid interface. J. Am. Chem. Soc. 121, 4563–4567 (1999).
Cerius2 version 2.0 (Molecular Simulations Inc., Cambridge, UK, 1998).
Gidalevitz,D. et al. Angew. Chem. Int. Edn Engl. 36, 955–959 (1997).
Acknowledgements
L.W.-S. and H.F.L. acknowledge support from ROPA awards.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davey, R., Williams-Seton, L., Lieberman, H. et al. Stabilizing a solid–solid interface with a molecular-scale adhesive. Nature 402, 797–799 (1999). https://doi.org/10.1038/45527
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/45527
This article is cited by
-
Anion-switchable supramolecular gels for controlling pharmaceutical crystal growth
Nature Chemistry (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.