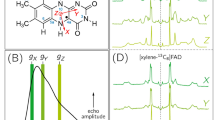
Abstract
The participation of tyrosine in the oxidation of water by photosystem II, a multisubunit enzyme complex involved in plant photosynthesis, exemplifies the significant role amino-acid side chains play in oxidation/reduction reactions in proteins1. The influence of the surrounding protein on the properties of amino-acid radicals and the attributes necessary for electron transfer are not well understood. Here we report that modifications of reaction centres from the purple bacterium Rhodobacter sphaeroides result in the generation of a tyrosyl radical in a manner similar to that of photosystem II. Our design incorporates a highly oxidizing bacteriochlorophyll dimer possessing a midpoint potential of more than 0.8 V, which was achieved by altering its local environment. After substitution of tyrosine residues at positions corresponding to the tyrosyl radicals of photosystem II, optical and electron paramagnetic resonance spectra showed changes consistent with oxidation of the tyrosine. The properties of this reaction include initiation by light and coupling to proton transfer, most probably to residues near the tyrosines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sigel,H. & Sigel,A. (eds) Metalloenzymes Involving Amino-Acid Residue and Related Radicals (Dekker, New York, 1994).
Diner,B. A. & Babcock,G. T. in Oxygenic Photosynthesis (eds Ort, D. R. & Yocum, C. F.) 213–247 (Kluwer, Dordrecht, 1996).
Rhee,K.-H. et al. Two-dimensional structure of plant photosystem II at 8-Å resolution. Nature 389, 522–526 (1997).
Allen,J. P. & Williams,J. C. Photosynthetic reaction centers. FEBS Lett. 438, 5–9 (1998).
Lin,X. et al. Specific alteration of the oxidation potential of the electron donor in reaction centers from Rhodobacter sphaeroides. Proc. Natl Acad. Sci. USA 91, 10265–10269 (1994).
Nagarajan,V., Parson,W. W., Davis,D. & Schenck,C. C. Kinetics and free energy gaps of electron-transfer reactions in Rhodovacter sphaeroides reaction centers. Biochemistry 32, 12324–12336 (1993).
Verméglio,A. & Clayton,R. K. Kinetics of electron transfer between the primary and the secondary electron acceptor in reaction centers from Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta 461, 159–165 (1977).
Diner,B. A. et al. in Photosynthesis: From Light to Biosphere Vol. II (ed. Mathis, P.) 229–234 (Kluwer, Dordrecht, 1995).
McElroy,J. D., Feher,G. & Mauzerall,D. C. Characterization of primary reactants in bacterial photosynthesis. Biochim. Biophys. Acta 267, 363–374 (1972).
Isaacson,R. A., Lendzian,F., Abresch,E. C., Lubitz,W. & Feher,G. Electronic structure of Q-A in reaction centers from Rhodobacter sphaeroides. Biophys. J. 69, 311–322 (1995).
Tang,X.-S., Chisholm,D. A., Dismukes,G. C., Brudvig,G. W. & Diner,B. A. Spectroscopic evidence from site-directed mutants of Synechocystis PCC6803 in favor of a close interaction between histidine 189 and redox-active tyrosine 160, both of polypeptide D2 of the photosystem II reaction center. Biochemistry 32, 13742–13748 (1993).
Hoganson,C. W., Sahlin,M., Sjoberg,B.-M. & Babcock,G. T. Electron magnetic resonance of the tyrosyl radical in ribonucleotide reductase from Escherichia coli. J. Am. Chem. Soc. 118, 4672–4679 (1996).
Stubbe,J. & van der Donk,W. A. Protein radicals in enzyme catalysis. Chem. Rev. 98, 705–762 (1998).
Tommos,C. et al. Modified EPR spectra of the tyrosineD radical in photosystem II in site-directed mutants of Synechocystis sp. PCC 6803: identification of side chains in the immediate vicinity of tyrosineD on the D2 protein. Biochemistry 32, 5436–5441 (1993).
Roffey,R. A., van Wijk,K. J., Sayre,T. S. & Styring,S. Spectroscopic characterization of tyrosine-Z in histidine 190 mutants of the D1 protein in photosystem II (PSII) in Chlamydomonas reinhardtii. J. Biol. Chem. 269, 5115–5121 (1994).
Manna,P., LoBrutto,R., Eijckelhoff,C., Dekker,J. P. & Vermaas,W. Role of Arg180 of the D2 protein in photosystem II structure and function. Eur. J. Biochem. 251, 142–154 (1998).
Un,S., Tang,X. S. & Diner,B. A. 245 GHz high-field EPR study of tyrosine-Do and tyrosine-Zo in mutants of photosystem II. Biochemistry 35, 679–684 (1996).
Hoganson,C. W. & Babcock,G. T. A metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science 277, 1953–1956 (1997).
Gilchrist,M. L. Jr, Ball,J. A., Randall,D. W. & Britt,R. D. Proximity of the manganese cluster of photosystem II to the redox-active tyrosine YZ. Proc. Natl Acad. Sci. USA 92, 9545–9549 (1995).
Hoganson,C. W. et al. A hydrogen-atom abstraction model for the function of YZ in photosynthetic oxygen evolution. Photosynthesis Res. 46, 177–184 (1995).
Tang,X.-S., Randall,D. W., Force,D. A., Diner,B. A. & Britt,R. D. Manganese-tyrosine interaction in the photosystem II oxygen-evolving complex. J. Am. Chem. Soc. 118, 7638–7639 (1996).
Mamedov,F., Sayre,R. T. & Styring,S. Involvement of Histidine 190 on the D1 protein in electron/proton transfer reactions on the donor side of photosystem II. Biochemistry 37, 14245–14256 (1998).
Hays,A.-M. A., Vassiliev,I. R., Golbeck,J. H. & Debus,R. J. Role of D1-His190 in proton-coupled electron transfer reactions in photosystem II: a chemical complementation study. Biochemistry 37, 11332–11365 (1998).
Ahlbrink,R. et al. Function of tyrosine Z in water oxidation by photosystem II: electrostatical promotor instead of hydrogen abstractor. Biochemistry 37, 1131–1142 (1998).
Blankenship,R. E. & Hartman,H. The origin and evolution of oxygenic photosynthesis. Trends Biol. Sci. 23, 94–97 (1998).
Feher,G., Allen,J. P., Okamura,M. Y. & Rees,D. C. Structure and function of bacterial photosynthetic reaction centres. Nature 339, 111–116 (1989).
Acknowledgements
We thank X. Nguyen, C. Magee, J. McCulley and N. Boruck for their assistance with this project, and W. Lubitz and F. Lendzian for preliminary EPR measurements. This work was supported by grants from the NSF and USDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kálmán, L., LoBrutto, R., Allen, J. et al. Modified reaction centres oxidize tyrosine in reactions that mirror photosystem II. Nature 402, 696–699 (1999). https://doi.org/10.1038/45300
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/45300
This article is cited by
-
De novo protein design of photochemical reaction centers
Nature Communications (2022)
-
High catalytic activity of gold nanoparticle-templated, tyrosine-rich peptide self-assemblies for 3,3′,5,5′-tetramethylbenzidine oxidation in the absence of hydrogen peroxide
Reaction Kinetics, Mechanisms and Catalysis (2019)
-
Electronic structure of the Mn-cofactor of modified bacterial reaction centers measured by electron paramagnetic resonance and electron spin echo envelope modulation spectroscopies
Photosynthesis Research (2014)
-
The evolutionary pathway from anoxygenic to oxygenic photosynthesis examined by comparison of the properties of photosystem II and bacterial reaction centers
Photosynthesis Research (2011)
-
Electronic Structure of Fe3+ at a Metal-Binding Site Introduced in Modified Bacterial Reaction Centers
Applied Magnetic Resonance (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.