Abstract
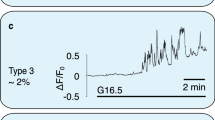
The hormone insulin is stored in secretory granules and released from the pancreatic β-cells by exocytosis1. In the consensus model of glucose-stimulated insulin secretion, ATP is generated by mitochondrial metabolism, promoting closure of ATP-sensitive potassium (KATP) channels, which depolarizes the plasma membrane2,3. Subsequently, opening of voltage-sensitive Ca2+ channels increases the cytosolic Ca2+ concentration ([Ca2+]c) which constitutes the main trigger initiating insulin exocytosis1,3,4. Nevertheless, the Ca2+ signal alone is not sufficient for sustained secretion. Furthermore, glucose elicits a secretory response under conditions of clamped, elevated [Ca2+]c (refs 5, 6). A mitochondrial messenger must therefore exist which is distinct from ATP7,8. We have now identified this as glutamate. We show that glucose generates glutamate from β-cell mitochondria. A membrane-permeant glutamate analogue sensitizes the glucose-evoked secretory response, acting downstream of mitochondrial metabolism. In permeabilized cells, under conditions of fixed [Ca2+]c, added glutamate directly stimulates insulin exocytosis, independently of mitochondrial function. Glutamate uptake by the secretory granules is likely to be involved, as inhibitors of vesicular glutamate transport suppress the glutamate-evoked exocytosis. These results demonstrate that glutamate acts as an intracellular messenger that couples glucose metabolism to insulin secretion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wollheim,C. B., Lang,J. & Regazzi,R. The exocytotic process of insulin secretion and its regulation by Ca2+ and G-proteins. Diabetes Rev. 4, 276–297 (1996).
Matschinsky,F. M. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45, 223–241 (1996).
Ashcroft,F. M. et al. Stimulus-secretion coupling in pancreatic beta cells. J. Cell Biochem. 55, 54–65 (1994).
Lang,J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 259, 3–17 (1999).
Gembal,M., Gilon,P. & Henquin,J. C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. J. Clin. Invest. 89, 1288–1295 (1992).
Sato,Y., Aizawa,T., Komatsu,M., Okada,N. & Yamada,T. Dual functional role of membrane depolarization/Ca2+ influx in rat pancreatic B-cell. Diabetes 41, 438–443 (1992).
Maechler,P., Kennedy,E. D., Pozzan,T. & Wollheim,C. B. Mitochondrial activation directly triggers the exocytosis of insulin in permeabilized pancreatic β-cells. EMBO J. 16, 3833–3841 (1997).
Maechler,P., Kennedy,E. D., Wang,H. & Wollheim,C. B. Desensitization of mitochondrial Ca2+ and insulin secretion responses in the beta cell. J. Biol. Chem. 273, 20770–20778 (1998).
Kennedy,E. D. et al. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J. Clin. Invest. 98, 2524–2538 (1996).
Duchen,M. R. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. (Lond.) 516, 1–17 (1999).
Fisher,H. F. L-glutamate dehydrogenase from bovine liver. Meth. Enzymol. 113, 16–27 (1985).
Janjic,D. et al. Improved insulin secretion of cryopreserved human islets by antioxidant treatment. Pancreas 13, 166–172 (1996).
Sener,A. et al. Insulinotropic action of glutamic acid dimethyl ester. Am. J. Physiol. 267, E573–E584 (1994).
Eto,K. et al. Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283, 981–985 (1999).
Maechler,P., Wang,H. & Wollheim,C. B. Continuous monitoring of ATP levels in living insulin secreting cells expressing cytosolic firefly luciferase. FEBS Lett. 422, 328–332 (1998).
Johnson,R. G. Jr Proton pumps and chemiosmotic coupling as a generalized mechanism for neurotransmitter and hormone transport. Ann. N. Y. Acad. Sci. 493, 162–177 (1987).
Maycox,P. R., Deckwerth,T., Hell,J. W. & Jahn,R. Glutamate uptake by brain synaptic vesicles. Energy dependence of transport reconstitution in proteoliposomes. J. Biol. Chem. 263, 15423–15428 (1988).
Hutton,J. C. The internal pH and membrane potential of the insulin-secretory granule. Biochem. J. 204, 171–178 (1982).
Orci,L. et al. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 103, 2273–2281 (1986).
Breckenridge,L. J. & Almers,W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature 328, 814–817 (1987).
Ozkan,E. D. & Ueda,T. Glutamate transport and storage in synaptic vesicles. Jpn. J. Pharmacol. 77, 1–10 (1998).
Roisin,M. P., Scherman,D. & Henry,J. P. Synthesis of ATP by an artificially imposed electrochemical proton gradient in chromaffin granule ghosts. FEBS Lett. 115, 143–147 (1980).
Roseth,S., Fykse,E. M. & Fonnum,F. Uptake of L-glutamate into rat brain synaptic vesicles: effect of inhibitors that bind specifically to the glutamate transporter. J. Neurochem. 65, 96–103 (1995).
Scheenen,W. J., Wollheim,C. B., Pozzan,T. & Fasolato,C. Ca2+ depletion from granules inhibits exocytosis. A study with insulin-secreting cells. J. Biol. Chem. 273, 19002–19008 (1998).
Churcher,Y. & Gomperts,B. D. ATP-dependent and ATP-independent pathways of exocytosis revealed by interchanging glutamate and chloride as the major anion in permeabilized mast cells. Cell Regul. 1, 337–346 (1990).
Jena,B. P. et al. Gi regulation of secretory vesicle swelling examined by atomic force microscopy. Proc. Natl Acad. Sci. USA 94, 13317–13322 (1997).
Hyder,F. et al. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc. Natl Acad. Sci. USA 93, 7612–7617 (1996).
Stanley,C. A. et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N. Engl. J. Med. 338, 1352–1357 (1998).
Cho,S. W., Lee,J. & Choi,S. Y. Two soluble forms of glutamate dehydrogenase isoproteins from bovine brain. Eur. J. Biochem. 233, 340–346 (1995).
Acknowledgements
We thank C. Bartley and G. Chaffard for technical assistance, J. Lou, J. Oberholzer and P. Morel (Department of Surgery, University Hospital of Geneva) for supplying human islets, A. Valeva (Institute of Medical Microbiology, University of Mainz) for α-toxin and T. Pozzan, P. Antinozzi and H. Ishihara for discussions. This study was supported by the Swiss National Science Foundation and the AETAS Foundation (Geneva).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maechler, P., Wollheim, C. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689 (1999). https://doi.org/10.1038/45280
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/45280
This article is cited by
-
Pancreatic β-cell glutaminase 2 maintains glucose homeostasis under the condition of hyperglycaemia
Scientific Reports (2023)
-
Luseogliflozin preserves the pancreatic beta-cell mass and function in db/db mice by improving mitochondrial function
Scientific Reports (2022)
-
Metabolism-secretion coupling in glucose-stimulated insulin secretion
Diabetology International (2022)
-
Mechanisms controlling pancreatic islet cell function in insulin secretion
Nature Reviews Molecular Cell Biology (2021)
-
Mitochondrial hydrogen peroxide positively regulates neuropeptide secretion during diet-induced activation of the oxidative stress response
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.