Abstract
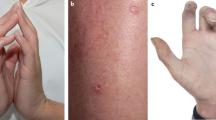
Originally described in 1964 as an idiopathic dermatosis occurring in women, Sweet's syndrome (acute febrile neutrophilic dermatosis) is associated with malignancy, typically haematological. We report a case occurring in a man receiving radical radiotherapy for locally advanced prostate cancer. To our knowledge, prostate cancer associated with Sweet's syndrome has been reported only once previously in the absence of other concurrent solid or haematological malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Cohen PR, Kuzrock R . Sweets syndrome revisited: a review of disease concepts. Int J Dermatol 2003; 42: 761–778.
Bourke JF, Keohane S, Long CC, Kemmett D, Davies M, Zaki I et al. Sweets syndrome and malignancy in the UK. Br J Dermatol 1997; 137: 609–613.
Cohen PR, Talpaz M, Kuzrock R . Malignancy-associated Sweet's syndrome: review of the world literature. J Clin Oncol 1988; 6: 1887–1897.
Cohen PR, Holder WR, Tucker SB, Kono S, Kuzrock R . Sweet Syndrome in patients with solid malignancy. Cancer 1993; 72: 2723–2731.
Dyall-Smith D, Billson V . Sweet's syndrome associated with adenocarcinoma of prostate. Australas J Dermatol 1988; 29: 25–27.
Leibowitz MR, Rippey JJ, Bezwoda WR, Carman HA . Unusual aspects of febrile neutrophilic dermatosis (Sweet's syndrome). Afr Med J 1982; 62: 375–378.
Barnadas MA, Sitjas D, Brunet S, Puig J, de Moragas JM . Acute febrile neutrophilic dermatosis (Sweet's syndrome) associated with prostate adenocarcinoma and a myelodysplastic syndrome. Int J Dermatol 1992; 31: 647–648.
Hussein KA, Al-Sabah H, Alsaleh QA . Sweet's syndrome (acute febrile neutrophilic dermatosis) associated with adenocarcinoma prostate and transitional cell carcinoma of urinary bladder. J Eur Acad Dermatol Venereol 2005; 19: 597–599.
Dawe SA, Phillips R, Porter W, Francis NA, Bunker C . Sweet's syndrome as a complication of radiotherapy for squamous carcinoma of the pharynx. Br J Dermatol 2003; 149: 884.
Van der Meij EH, Epstein JB, Hay J, Ho V, Lerner K . Sweet's syndrome in a patient with oral cancer associated with radiotherapy. Eur J Cancer B Oral Oncol 1996; 32B: 133–161.
Vergara G, Vargas-Machuca I, Pastor MA, Farina MC, Martin L, Requena L . Localisation of Sweet's syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol 2003; 49: 907–909.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glendenning, J., Khoo, V. Sweet's syndrome in prostate cancer. Prostate Cancer Prostatic Dis 11, 397–398 (2008). https://doi.org/10.1038/sj.pcan.4501029
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4501029