Abstract
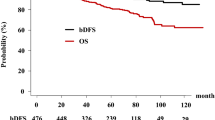
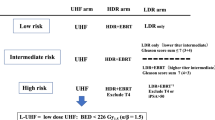
The role of neoadjuvant androgen deprivation (NAD) in high-risk prostate cancer patients receiving high-dose radiotherapy (RT) remains unstudied. To evaluate the effect of a course of NAD, we reviewed the experiences of three institutions treating these patients with combined RT and high-dose rate brachytherapy (HDR). Of 1260 prostate cancer patients with high-risk features (pretreatment prostate-specific antigen (PSA) ⩽10, Gleason Score (GS) ⩽7, or T stage ⩽T2b), 560 received no NAD (n=308) or NAD for ⩾6 months (n=252). Median dose to the prostate from RT and HDR was 42 and 23 Gy, respectively. Average total biologic equivalent prostate dose was >100 Gy (α/β=1.2). Median follow-up was 4.3 years. Pretreatment characteristics were similar on χ2 tables for all 560 patients treated with or without NAD including pretreatment PSA (P=0.11), GS (P=0.4), and clinical T stage (P=0.2). Outcomes worsened for patients receiving NAD (5-year distant metastasis (DM) 10 vs 5% (P=0.04); cause-specific survival (CSS), 93 vs 98% (P=0.005)). Higher 5-year DM rates and lower CSS occurred in NAD patients with a GS between 8 and 10 (n=112 (P=0.03, P=0.02)), pretreatment PSA⩽15 (n=136 (P=0.03, P=0.008)), and palpable disease ⩽T2a (n=434 (P=0.04, P=0.02)). The only two significant risk factors for DM on Cox multivariate analysis were GS (P=0.003, HR 2.8) and NAD (P=0.03, HR 2.7). AD given before definitive high-dose RT did not benefit prostate cancer patients with intermediate- and high-risk features. We favor the use of concurrent/adjuvant AD over prolonged NAD for prostate cancer patients for whom AD is clinically indicated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360: 103–106.
D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW . 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004; 292: 821–827.
Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 2003; 21: 3972–3978.
Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001; 50: 1243–1252.
Pilepich MV, Winter K, Lawton C, Krisch RE, Wolkov H, Movsas B et al. Androgen suppression adjuvant to radiotherapy in carcinoma of the prostate. long-term results of phase III RTOG study 85-31. Int J Radiat Oncol Biol Phys 2003; 57: s172 (Abstract).
Roach III M, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003; 21: 1904–1911.
Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L et al. Report of a multicenter Canadian phase III randomized trial of 3 vs 8 months neoadjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 60: 15–23.
Pollack A, Zagars GK, Starkschall G, Antolak JA, Lee JJ, Huang E et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 2002; 53: 1097–1105.
Zietman AL, DeSilvio M, Slater JD, Rossi C, Yonemoto L, Slater JM et al. A randomized trial comparing conventional dose (70.2 GyE) and high-dose (79.2 GyE) conformal radiation in early stage adenocarcinoma of the prostate: results of an interim analysis of PROG 95-09. Int J Radiat Oncol Biol Phys 2004; 60: S131–S132, (Abstract).
Zietman AL, DeSilvio ML, Slater JD, Rossi Jr CJ, Miller DW, Adams JA et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 2005; 294: 1233–1239.
Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 2003; 21: 3972–3978.
Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L et al. Report of a multicenter Canadian phase III randomized trial of 3 vs 8 months neoadjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 60: 15–23.
Martinez A, Galalae R, Gonzalez J, Mitchell C, Gustafson G, Kovacs G . No apparent benefit at 5 years from a course of neoadjuvant/concurrent androgen deprivation for patients with prostate cancer treated with a high total radiation dose. J Urol 2003; 170: 2296–2301.
Martinez A, Vargas C, Demanes J, Galalae R, Bertermann H, Rodriguez RR et al. Detrimental outcome of short term hormonal treatment and high dose radiation therapy (EBRT plus HDR) when compared to EBRT plus HDR alone for high risk prostate patients. Int J Radiat Oncol Biol Phys 2004; 60: S469 (Abstract).
Agency for Health Care Policy and Research. Relative Effectiveness and Cost-Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostatic Cancer. http://www.ahrqgov/clinic/epcsums/prossummhtm.; 1999. 7-14-2004. (GENERIC).
American Joint Committee on Cancer. Prostate. In: Beahrs OH, Henson DE, Hutter RVP, Kennedy BJ (eds). AJCC Cancer Staging Manual. Lippincott-Raven: Philadelphia, 1992; pp 181–186.
Martinez A, Gonzalez J, Spencer W, Gustafson G, Kestin L, Kearney D et al. Conformal high dose rate brachytherapy improves biochemical control and cause specific survival in patients with prostate cancer and poor prognostic factors. J Urol 2003; 169: 974–979.
Vicini FA, Vargas C, Edmundson G, Kestin L, Martinez A . The role of high-dose rate brachytherapy in locally advanced prostate cancer. Semin Radiat Oncol 2003; 13: 98–108.
Demanes DJ, Rodriguez RR, Altieri GA . High dose rate prostate brachytherapy: the California Endocurietherapy (CET) method. Radiother Oncol 2000; 57: 289–296.
Galalae RM, Martinez A, Mate T, Mitchell C, Edmundson G, Nuernberg N et al. Long-term outcome by risk factors using conformal high-dose-rate brachytherapy (HDR-BT) boost with or without neoadjuvant androgen suppression for localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 58: 1048–1055.
Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP . Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002; 52: 6–13.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life-tables. J R Stat Soc B 1972; 34: 187–220.
Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P et al. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001; 50: 1243–1252.
Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360: 103–106.
Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 2003; 21: 3972–3978.
Roach III M, DeSilvio M, Lawton C, Uhl V, Machtay M, Seider MJ et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003; 21: 1904–1911.
D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW . 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 2004; 292: 821–827.
Crook J, Ludgate C, Malone S, Lim J, Perry G, Eapen L et al. Report of a multicenter Canadian phase III randomized trial of 3 vs 8 months neoadjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004; 60: 15–23.
Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 1993; 11: 336–344.
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002; 20: 3054–3060.
Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005; 23: 5883–5891.
Soloway MS, Pareek K, Sharifi R, Wajsman Z, McLeod D, Wood Jr DP et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol 2002; 167: 112–116.
Nguyen PL, Whittington R, Koo S, Schultz D, Cote KB, Loffredo M et al. The impact of a delay in initiating radiation therapy on prostate-specific antigen outcome for patients with clinically localized prostate cancer. J Clin Oncol 2004; 22, (Abstract) 4503.
Nam RK, Jewet MA, Krahn MD, Robinette MA, Tsihlias J, Toi A et al. Short-Term Delays in Surgical Therapy for Clinically Localized Prostate Cancer May Reduce Cancer Cure Rates. American Urologic Association Meeting 2002. 2002. (GENERIC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the ASTRO Meeting in Atlanta, Georgia 2004.
Rights and permissions
About this article
Cite this article
Vargas, C., Martínez, A., Galalae, R. et al. High-dose radiation employing external beam radiotherapy and high-dose rate brachytherapy with and without neoadjuvant androgen deprivation for prostate cancer patients with intermediate- and high-risk features. Prostate Cancer Prostatic Dis 9, 245–253 (2006). https://doi.org/10.1038/sj.pcan.4500882
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500882
Keywords
This article is cited by
-
High-risk prostate cancer—classification and therapy
Nature Reviews Clinical Oncology (2014)