Abstract
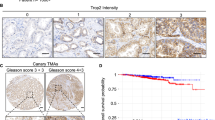
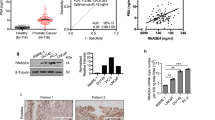
Functional expression of voltage-gated sodium channel α-subunits (VGSCαs), specifically Nav1.7, is associated with strong metastatic potential in prostate cancer (CaP) in vitro. Furthermore, VGSC activity in vitro directly potentiates processes integral to metastasis. To investigate VGSCα expression in CaP in vivo, immunohistochemistry and real-time PCR were performed on human prostate biopsies (n>20). VGSCα immunostaining was evident in prostatic tissues and markedly stronger in CaP vs non-CaP patients. Importantly, RT-PCRs identified Nav1.7 as the VGSCα most strikingly upregulated (∼20-fold) in CaP, and the resultant receiver-operating characteristics curve demonstrated high diagnostic efficacy for the disease. It is concluded that VGSCα expression increases significantly in CaP in vivo and that Nav1.7 is a potential functional diagnostic marker.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Foster CS et al. The cellular and molecular basis of prostate cancer. Br J Urol Int 1999; 83: 171–194.
Graves HC . Nonprostatic sources of prostate-specific antigen: a steroid hormone-dependent phenomenon? Clin Chem 1995; 41: 7–9.
Smith MR, Biggar S, Hussain M . Prostate-specific antigen messenger RNA is expressed in non-prostate cells: implications for detection of micrometastases. Cancer Res 1995; 55: 2640–2644.
Carroll P et al. Prostate-specific antigen best practice policy—Part I: early detection and diagnosis of prostate cancer. Urology 2001; 57: 217–224.
Carroll P et al. Prostate-specific antigen best practice policy—Part II: prostate cancer staging and post-treatment follow-up. Urology 2001; 57: 225–229.
Law M . Screening without evidence of efficacy. BMJ 2004; 328: 301–302.
de Kok JB et al. DD3 (PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res 2002; 62: 2695–2698.
Chakravarti A, Zhai GG . Molecular and genetic prognostic factors of prostate cancer. World J Urol 2003; 21: 265–274.
Varambally S et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002; 419: 624–629.
Grimes JA et al. Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett 1995; 369: 290–294.
Laniado ME et al. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasiveness in vitro. Am J Pathol 1997; 150: 1213–1221.
Smith P et al. Sodium channel protein expression enhances the invasiveness of rat and human prostate cancer cells. FEBS Lett 1998; 423: 19–24.
Fraser SP et al. Tetrodotoxin suppresses morphological enhancement of the metastatic MAT-LyLu rat prostate cancer cell line. Cell Tiss Res 1999; 295: 505–512.
Mycielska ME, Dye J, Stepien E, Djamgoz MBA . Substrate influences voltage-gated Na+ channel expression in strongly metastatic rat prostate cancer cell line. J Physiol 2004: 557P: C85.
Fraser SP et al. Contribution of functional voltage-gated Na+ channel expression to cell behaviours involved in the metastatic cascade in rat prostate cancer. I Lateral motility. J Cell Physiol 2003; 195: 479–487.
Djamgoz MBA et al. Directional movement of rat prostatic cancer cells in direct-current electric field: involvement of voltage-gated Na+ channel activity. J Cell Sci 2001; 114: 2697–2705.
Mycielska ME, Djamgoz MBA . Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci 2004; 117: 1631–1639.
Mycielska ME, Fraser SP, Szatkowski M, Djamgoz MBA . Contribution of functional voltage-gated Na+ channel expression to cell behaviours involved in the metastatic cascade in rat prostate cancer. II Secretory membrane activity. J Cell Physiol 2003; 195: 461–469.
Bennett ES, Smith BA, Harper JM . Voltage-gated Na+ channels confer invasive properties to human prostate cancer cells. Pflugers Arch 2004; 447: 908–914.
Goldin AL et al. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci USA 1986; 83: 7503–7507.
Isom LL et al. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 1992; 256: 839–842.
Isom LL et al. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 1995; 83: 433–442.
Goldin AL et al. Nomenclature of voltage-gated sodium channels. Neuron 2000; 28: 365–368.
Diss JKJ et al. Expression profiles of voltage-gated Na+ channel α-subunit genes in rat and human prostate cancer cell lines. Prostate 2001; 48: 165–178.
Smith P, Rhodes NP, Ke Y, Foster CS . Relationship between upregulated oestrogen receptors and expression of growth factors in cultured, human, prostatic stromal cells exposed to estradiol or dihydrotestosterone. Prostate Cancer Prostate Dis 2004; 7: 57–62.
Foster CS, Sakr WA . Proliferative lesions of the prostate that mimic carcinoma. Curr Diagn Pathol 2001; 7: 194–212.
Dugandzija-Novakovic S, Koszowski AG, Levinson SR, Shrager P . Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J Neurosci 1995; 15: 492–503.
Pei L et al. PRC17, a novel oncogene encoding a Rab GTPase-activating protein, is amplified in prostate cancer. Cancer Res 2002; 62: 5420–5424.
Stephan C et al. Quantitative analysis of kallikrein 15 gene expression in prostate tissue. J Urol 2003; 169: 361–364.
Stephan C et al. Hepsin is over expressed in and a new candidate for a prognostic indicator in prostate cancer. J Urol 2004; 171: 187–191.
Epner DE, Coffey DS . There are multiple forms of glyceraldehyde-3-phosphate dehydrogenase in prostate cancer cells and normal prostate tissue. Prostate 1996; 28: 372–378.
Rondinelli RH, Epner DE, Tricoli JV . Increased glyceraldehydes-3-phosphate dehydrogenase gene expression in late pathological stage human prostate cancer. Prostate Cancer Prostate Dis 1997; 1: 66–72.
Kreuzer KA et al. Highly sensitive and specific fluorescence reverse transcription-PCR assay for the pseudogene-free detection of beta-actin transcripts as quantitative reference. Clin Chem 1999; 45: 297–300.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) Method. Methods 2001; 25: 402–408.
Bostwick DG, Pacelli A, Lopez-Beltran A . Molecular biology of prostatic intraepithelial neoplasia. Prostate 1996; 29: 117–134.
Abdul M, Hoosein N . Voltage-gated sodium ion channels in prostate cancer: expression and activity. Anticancer Res 2002; 22: 1727–1730.
Abdul M, Hoosein N . Expression and activity of potassium ion channels in human prostate cancer. Cancer Lett 2002; 186: 99–105.
Fraser SP et al. Predominant expression of Kv1.3 voltage-gated K+ channel subunit in rat prostate cancer cell lines: electrophysiological, pharmacological and molecular characterisation. Pflugers Arch 2003; 446: 559–571.
Djamgoz MBA . Voltage-gated sodium channel activity and metastasis: a novel approach to understanding the pathophysiology of prostate cancer. J Physiol 1998; 513: 21S–22S.
Klugbauer N, Lacinova L, Flockerzi V, Hofmann F . Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J 1995; 14: 1084–1090.
Cummins TR, Howe JR, Waxman SG . Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J Neurosci 1998; 18: 9607–9619.
Toledo-Aral JJ et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA 1997; 94: 1527–1532.
Toledo-Aral JJ, Brehm P, Halegoua S, Mandel G . A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron 1995; 14: 607–611.
Barton J, Blackledge G, Wakeling A . Growth factors and their receptors: new targets for prostate cancer therapy. Urology 2001; 58: 114–122.
Krasowska M et al. Patterning of endocytic vesicles and its control by voltage-gated Na+ channel activity in rat prostate cancer cells: fractal analyses. Eur Biophys J 2004; 33: 535–542.
Abdul M, Hoosein N . Inhibition by anticonvulsants of prostate-specific antigen and interleukin-6 secretion by human prostate cancer cells. Anticancer Res 2001; 21: 2045–2048.
Montano X, Djamgoz MBA . Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett 2004; 571: 1–8.
Anderson JD et al. Voltage-gated sodium channel blockers as cytostatic inhibitors of the androgen-independent prostate cancer cell line PC-3. Mol Cancer Ther 2003; 2: 1149–1154.
Sikes RA et al. Therapeutic approaches targeting prostate cancer progression using novel voltage-gated ion channel blockers. Clin Prostate Cancer 2003; 2: 181–187.
Trepel JB . Ion channels as molecular targets in prostate cancer. Clin Prostate Cancer 2003; 2: 188–189.
Acknowledgements
This study was supported by PCaSO (Prostate Cancer Network) and the Pro-Cancer Research Fund (PCRF). We thank Dr Graham Wilkin for use of OPTIMAS 5.2 image analysis system and Professor Norman Maitland (supported by Yorkshire Cancer Research) for the PNT2-C2 cell line.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diss, J., Stewart, D., Pani, F. et al. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis 8, 266–273 (2005). https://doi.org/10.1038/sj.pcan.4500796
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500796
Keywords
This article is cited by
-
Lidocaine Inhibits Rat Prostate Cancer Cell Invasiveness and Voltage-Gated Sodium Channel Expression in Plasma Membrane
The Journal of Membrane Biology (2024)
-
Genomic analyses reveal SCN7A is associated with the prognosis of esophageal squamous cell carcinoma
Esophagus (2022)
-
Imaging the transmembrane and transendothelial sodium gradients in gliomas
Scientific Reports (2021)
-
Anti-metastatic effect of ranolazine in an in vivo rat model of prostate cancer, and expression of voltage-gated sodium channel protein in human prostate
Prostate Cancer and Prostatic Diseases (2019)
-
The invasiveness of human cervical cancer associated to the function of NaV1.6 channels is mediated by MMP-2 activity
Scientific Reports (2018)