Abstract
Purpose: To improve the rate of full continence in our patients, we performed, since June 1997, a careful preparation of the distally intraprostatic part of the membranous urethra to obtain a long urethral stump for the vesicourethral anastomosis.
Patients and methods: In all, 610 patients without (group 1) and 403 patients with (group 2) a long intraprostatic stump of the urethra were asked by a self-administered questionnaire about their continence status. The rate of positive surgical margins were compared as a marker of local tumour control.
Results: Full continence (no pads) was achieved in 76.02% in group 1 and in 88.84%, of all patients in group 2. Stress incontinence (SIC) I° was found in 12.46% and 7.44% respectiveley, SIC II° was noted in 8.69 and 3.72% and complete incontinence was seen in 2.79% in group 1 and in two patients (0.5%) in group 2. Also the time to reach the final continence status was statistically and highly significantly (P<0.001) shortened. The rate of positive margins decreased in group 2, despite intraprostatic preparation.
Conclusions: The preparation of a long, partially intraprostatic portion of the membranous urethra for vesicourethral anastomosis in radical retropubic prostatectomy leads to a statistically highly significant improvement of full continence and earlier continence in prostate cancer patients without compromising local tumour control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bates TS, Wright MP, Gillatt DA . Prevalence and impact of incontinence and impotence following total prostatectomy assessed anonymously by the ICS-male questionnaire. Eur Urol 1998; 33: 165–169.
Litwin MS et al. Urinary function and bother after radical prostatectomy or radiation for prostate cancer: a longitudinal, multivariate quality of life analysis from the Cancer of the Prostate Strategic Urologic Research Endeavour. J Urol 2000; 164: 1973–1977.
Walsh PC, Marschke P, Ricker D, Burnett AL . Patient reported urinary continence and sexual function after anatomic radical prostatectomy. Urology 2000; 55: 58–61.
Wei JT et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol 2000; 164: 744–748.
Stanford JL et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000; 283: 354–360.
Catalona WJ, Carvahal GF, Mager DE, Smith DS . Potency, continence and complication rates in 1870 consecutive radical retropubic prostatectomies. J Urol 1999; 162: 433–438.
Steiner MS, Morton RA, Walsh PC . Impact of anatomical radical prostatectomy on urinary continence. J Urol 1991; 145: 512–514.
Steiner MS . Continence preserving anatomic radical prostatectomy. Urology 2000; 55: 427–435.
Walsh PC, Marschke PL . Intussusception of the reconstructed bladder neck leads to earlier continence after radical prostatectomy. Urology 2002; 59: 934–938.
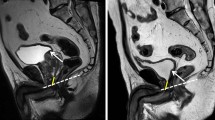
Coakley FV et al. Urinary incontinence after radical retropubic prostatectomy: relationship with membraneous urethral length on preoperative endorectal magnetic resonance imaging. J Urol 2002; 168: 1032–1035.
Myers RP . Radical prostatectomy: making it a better operation in the new millenium. Int J Urol 2001; 8: 9–14.
Maffezzini M et al. Recovery of urinary continence after retropubic radical prostatectomy. Results in 1000 patients. Arch Ital Urol Androl 2001; 73: 145–152.
Peyromaure M, Ravery V, Boccon-Gibbod L . The management of stress urinary incontinence after radical prostatectomy. BJU Int 2002; 90: 155–161.
Myers RP, Goellner JR, Cahill DR . Prostate shape, external striated urethral sphincter and radical prostatectomy: the apical dissection. J Urol 1987; 138: 543–550.
Myers RP . Male urethral sphincteric anatomy and radical prostatectomy. Urol Clin North Am 1991; 18: 211–227.
Salomon L et al. Location of positive surgical margins after retropubic, perineal and laparoscopic radical prostatectomy for organ-confined prostate cancer. Urology 2003; 61 (2): 386–390.
Salomon L et al. Prognostic consequences of the location of positive surgical margins in organ confined prostate cancer. Urol Int 2003; 70: 291–296.
Bianco FJ et al. Radical prostatectomy with bladder neck preservation: impact on positive margins. Eur Urol 2003; 43: 461–466.
Noldus J, Palisaar J, Huland H . Treatment of prostate cancer — the clinical use of radical prostatectomy. EAU Update Ser 2003; 1: 16–22.
Shah O et al. Positive surgical margins at radical prostatectomy: importance of the apical dissection. J Urol 2001; 165: 1943–1948.
Mc Neal JE et al. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Path 1990; 14: 240.
Epstein JI . Incidence and significance of positive surgical margins in radical retropubic prostatectomy specimens. Urol Clin North Am 1996; 23: 651.
Myers RP . Radical prostatectomy: making it a better operation in the new millenium. Int J Urol 2001; 8: 9–14.
Presti JC et al. Physiology of urinary incontinence after radical prostatectomy. J Urol 1990; 143: 975–978.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Randenborgh, H., Paul, R., Kübler, H. et al. Improved urinary continence after radical retropubic prostatectomy with preparation of a long, partially intraprostatic portion of the membraneous urethra: an analysis of 1013 consecutive cases. Prostate Cancer Prostatic Dis 7, 253–257 (2004). https://doi.org/10.1038/sj.pcan.4500726
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500726
Keywords
This article is cited by
-
Anterior fibromuscular stroma-preserved endoscopic enucleation of the prostate: a precision anatomical approach
World Journal of Urology (2022)
-
Feasibility and continence outcomes of extended prostatic urethral preservation during robot-assisted radical prostatectomy
Prostate Cancer and Prostatic Diseases (2020)
-
Development and validation of nomograms to predict the recovery of urinary continence after radical prostatectomy: comparisons between immediate, early, and late continence
World Journal of Urology (2014)
-
Morphology and dynamics of the male pelvic floor before and after retrourethral transobturator sling placement: first insight using MRI
World Journal of Urology (2013)
-
Effect of minimizing tension during robotic-assisted laparoscopic radical prostatectomy on urinary function recovery
World Journal of Urology (2013)