The rods in salamanders' retinas can co-opt a molecule derived from chlorophyll to detect red light.
Abstract
In the absence of a red-sensitive visual pigment, some deep-sea fish use a chlorophyll derivative in their green-sensitive rod cells in order to see deep-red light1,2,3. Here we show that living rods extracted from a salamander can also accumulate an exogenous chlorophyll derivative, chlorin e6, that renders them as sensitive to red light as they are to green. This vision enhancement by an unbleachable chlorophyll derivative might therefore be a general phenomenon in vertebrate photoreception.
Similar content being viewed by others
Main
The outer segments of rod cells are rich in rhodopsin: this visual pigment consists of retinal, a form of vitamin A that isomerizes when it absorbs light, and opsin, a protein that changes shape in response to retinal isomerization, which triggers a signal.
We measured the absorbance spectra of individual rods from tiger salamanders (Ambystoma tigrinum) by microspectrophotometry (for methods, see supplementary information). Absorbance peaked at wavelengths of 520 nm and 278 nm, owing to the retinal chromophore and the opsin, respectively.
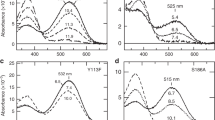
When rods were preincubated with chlorin (Fig. 1a), we found that chlorin targeted the rhodopsin-laden outer segments of the rods; it increased absorbance at 278 nm, and added peaks at 402 nm and 668 nm (Fig. 1b). The peak at 520 nm was unchanged, so if chlorin did bind to rhodopsin, it did so without influencing the retinal chromophore or the binding pocket that spectrally tunes rhodopsin. The slight increase in absorbance of 278-nm light indicates that chlorin may be perturbing aromatic residues on the surface of rhodopsin.
a, Structure of the tetrapyrrole chlorin e6. b, Averaged absorption from chlorin-treated (red trace; n = 5) and control (blue trace; n = 5) rods. The spectrum of treated rods is normalized to the 278-nm peak, and for untreated rods is scaled to match the 520-nm peak for treated rods. c, Rhodopsin bleaching from untreated rods (filled symbols; mean ± s.e.m.), aligned to a template fit7 to the spectral sensitivity of seven untreated rods (blue trace). The bleaching rate of rods with high chlorin content (open symbols) is enhanced at 668 nm but not at 560 nm; the rate mirrors the average absorption of such rods (dashed trace), showing that quanta absorbed by chlorin and by rhodopsin are equally effective at bleaching rhodopsin (for details, see supplementary information). d, Spectral sensitivity (circles; n = 3) increases at 661 and 672 nm in chlorin-treated rods and matches the normalized absorption of a set of rods treated with chlorin and measured microspectrophotometrically (red trace); blue trace is from c. Inset, response (in picoamperes, pA) of a chlorin-treated rod to dim flashes at 540 nm (blue; 6.4 × 10−1 photons μm−2) and at 672 nm (red; 22.3 photons μm−2).
We selected cells with the highest chlorin content (chlorin:rhodopsin ratio was 0.77 ± 0.22, mean ± s.e.m.; n = 3) to examine chlorin's effect on rhodopsin's sensitivity to light, measured by its bleaching rate. The bleaching rate was unchanged at 560 nm, a wavelength absorbed well by rhodopsin but poorly by chlorin. However, it was accelerated 180-fold at 668 nm, where chlorin absorbs strongly but rhodopsin absorbance is negligible (Fig. 1c).
This bleaching enhancement was tens to hundreds of times greater than the effect of chlorophyll derivatives on dragon fish (Malacosteus niger)2,3 and bovine4 rhodopsins. This shows that chlorophyll-sensitized vision is feasible. Adding 9-cis-retinal regenerated a light-sensitive pigment, irrespective of the bleaching conditions that had been used, indicating that chlorin-mediated rhodopsin bleaching was not due to a permanent, photo-induced degradation of opsin (for details, see supplementary information).
In electrical recordings of rods exposed to chlorin, responses to dim flashes were similar in form at all wavelengths (Fig. 1d; inset shows response to two wavelengths). This indicates that photons absorbed by chlorin or by the retinal chromophore directly induce the same active state in rhodopsin. Chlorin treatment increased rod sensitivity to red light (at 661 and 672 nm) by 15-fold (Fig. 1d). This enhancement was less than the 180-fold increase in bleaching rate found earlier, because the cells used for recordings were randomly chosen and contained about 20 times less chlorin (chlorin:rhodopsin ratio, 0.05 ± 0.01, mode of 61 rods ± s.e.m; n = 15). Nevertheless, in both microspectrophotometric and electrophysiological experiments, chlorin-assisted bleaching was almost as efficient as direct photoisomerization of the native chromophore (Fig. 1, and see supplementary information).
The mechanism behind the highly efficient energy transfer from chlorin to the retinal chromophore is unclear at this point. However, our results on an amphibian, together with findings from dragon fish1,2,3, bovine4, insect5 and prokaryotic6 rhodopsins, highlight a potential for spectral enhancement by accessory chromophores in all opsin-based photoreception systems.
References
Bowmaker, J. K., Dartnall, H. J. A. & Herring, P. J. J. Comp. Physiol. A 163, 685–698 (1988).
Douglas, R. H. et al. Nature 393, 423–424 (1998).
Douglas, R. H. et al. Vision Res. 39, 2817–2832 (1999).
Washington, I., Brooks, C., Turro, N. J. & Nakanishi, K. J. Am. Chem. Soc. 126, 9892–9893 (2004).
Kirschfeld, K. & Vogt, K. Vision Res. 26, 1771–1777 (1986).
Balashov, S. P. et al. Science 309, 2061–2064 (2005).
Govardovskii, V. I., Fyhrquist, N., Reuter, T., Kuzmin, D. G. & Donner, K. Visual Neurosci. 17, 509–528 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
supplementary figures, equations and methods (PDF 170 kb)
Rights and permissions
About this article
Cite this article
Isayama, T., Alexeev, D., Makino, C. et al. An accessory chromophore in red vision. Nature 443, 649 (2006). https://doi.org/10.1038/443649a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/443649a
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.