Abstract
Apoptosis is mediated by cysteine-dependent, aspartate-directed proteases of the caspase family that proteolyse strategic intracellular substrates to induce cell suicide. We describe here that engagement of apoptotic processes by Fas triggering or by staurosporine stimulation leads to the caspase-dependent inactivation of the nuclear factor kappa B (NF-κB) pathway after cleavage of IKK1 (IκB kinase 1) and NEMO (NF-κB essential modulator), which are needed to transduce NF-κB activation signals. In this study, we have analyzed in more detail, the role of NEMO cleavage, as NEMO, but not IKK1, is important for the pro-survival actions of NF-κB. We demonstrate that NEMO is cleaved after Asp355 to remove the last 64 C-terminal amino acids. This short form was unable to rescue NF-κB activation by tumor necrosis factor-α (TNF-α) when transfected in NEMO-deficient cells. Consequently, inactivation of NEMO resulted in an inhibition of the expression of antiapoptotic NF-κB-target genes coding for caspase inhibitors (cIAP-1, cIAP-2) or adaptors of the TNF receptor family. NEMO-deficient Jurkat cells transiently expressing a non-cleavable mutant of NEMO were less sensitive to TNF-α-induced apoptosis. Therefore, downmodulation of NF-κB activation via the proteolytic cleavage of NEMO could represent an amplification loop for apoptosis.
Similar content being viewed by others
Main
Nuclear factor kappa B (NF-κB) transcription factors are important regulators of cell death and survival. Five NF-κB members have been identified in mammals: p65/RelA, c-Rel, RelB, p50/NF-κB1 and p52/NF-κB2 that associate as dimers to bind specific target DNA sequences to regulate transcription.1 NF-κB is sequestered inactive in the cytosol by inhibitory subunit of the IκB family (IκB-α, IκB-β, IκB-γ, IκB-ɛ). Upon cell stimulation by a wide panel of external as well as internal stimuli,2 IκB molecules are phosphorylated on serine residues (32 and 36 for IκB-α), an event that triggers polyubiquitination and proteasome-mediated in situ degradation of the inhibitor. This serves to free NF-κB and to allow its translocation to the nucleus (reviewed in Scheidereit3). Phosphorylation is catalyzed by the I kappa B kinase (IKK) complex, comprised of two IκB specific kinases IκB kinase (IKK)1 and IKK2, and a scaffold protein IKKγ/NEMO (NF-κB essential modulator), which is required for the assembly of a functional IKK complex. IKK1 and IKK2 are closely related (52% homology) and form homo- and hetero-dimers, but regulate different signaling pathways. IKK2, which transduces inflammatory signals resulting in IκB-α phosphorylation, controls the canonical NF-κB activation pathway, while IKK1 regulates the processing of NF-κB2/p100 to mediate secondary lymphoid organogenesis (reviewed in Pomerantz and Baltimore4). The lack of NEMO results in disassembly of the IKK complex and a failure to transmit NF-κB activation signals via the classical pathway.5
IκB-α phosphorylation and degradation are rapid reactions. Within minutes of stimulation, free NF-κB dimers migrate to the nucleus to activate transcription of a wide array of target genes.2
Embryos knocked out for RelA/p65,6 IKK27 or NEMO8 die due to an uncontrolled tumor necrosis factor-α (TNF-α)-mediated apoptosis in the liver, demonstrating that NF-κB is required for survival. NF-κB interferes with various steps in apoptotic cascades. It regulates expression of genes for TNF receptor adaptors (TRAFs) that form amplifying loops to strengthen NF-κB activation. By inducing expression of c-FLIP, c-IAP-1 and c-IAP-2, NF-κB can dampen caspase activation. NF-κB can also heighten antiapoptotic influences at the mitochondrial level through expression of the Bcl-2 family members bfl-1/A1 and Bcl-xl9 (see Karin and Lin10 for review). Abnormal constitutive NF-κB activation is a characteristic of many cancerous cell lines and inhibition of NF-κB by pharmacological means or by expression of a super-repressor form of IκB-α, often leads to apoptosis or sensitizes cancer cells to the apoptotic action of TNF-α,11, 12 TRAIL,13 chemotherapeutic drugs,14, 15, 16 or phorbol myristate acetate (PMA),17 reviewed in Karin et al.18
Apoptotic pathways are intended to disrupt cellular architecture and functions. Besides the cleavage of structural proteins, caspases inactivate proteins involved in the transmission of antiapoptotic influences to amplify apoptotic reactions.19, 20 Key examples are protein kinases that transmit activation/survival signals such as fyn, lyn,21 zap-70, FAK, PKC θ and δ and Akt.19 Caspases also target several antiapoptotic proteins endowing some of them such as Bcl-2, Bcl-xl, with apoptotic functions after cleavage. The SRF (serum response factor),22 p65/NF-κB transcription factors23 involved in cell survival are also converted by cleavage into dominant negative forms and therefore act as pro-apoptotic proteins.
We demonstrate here that NEMO is proteolysed by cellular caspases during apoptosis. This event interferes with transmission of NF-κB activation signals and is proposed to represent a regulatory loop to enhance apoptosis.
Results
Induction of apoptosis down regulates NF-κB activation
Apoptosis of human Jurkat leukemic T cells was induced by engagement of the Fas receptor with the CH11 agonist antibody for 6 h. NF-κB activation was then triggered by 1 h stimulation with either TNF-α or the phorbol ester PMA. NF-κB DNA-binding activity was measured in total cell extracts by electrophoretic mobility shift assay (EMSA) (Figure 1a). NF-κB activation by either TNF-α (lane 2) or by PMA (lane 3), was strongly prevented by pretreatment with CH11 (lanes 5 and 6), which had no effect by itself (lane 4). As a control, it is shown (Figure 1b) that under the same conditions, the constitutive DNA-binding activity of the transcription factor Oct-1 was not affected by cell stimulation with PMA (lane 2) or TNF-α (lane 3) nor by Fas engagement alone (lane 4) or in combination with PMA (lane 5) or TNF-α (lane 6).
Anti-Fas antibody (CH11) inhibits NF-κB activation induced by TNF-α or PMA. (a and b) EMSA analysis of NF-κB and Oct-1 activation: Jurkat cells were incubated with anti-Fas antibody CH11 (100 ng/ml) for 5 h before stimulation with TNF-α (10 ng/ml) or PMA (10 ng/ml) for 1 h. DNA/protein complexes were separated by non-denaturing electrophoresis followed by autoradiography. (c) Jurkat cells were stimulated with z-VAD-FMK (50 μM) for 24 h before stimulation with CH11 (100 ng/ml) for 5 h followed by TNF-α (10 ng/ml) for 1 h. NF-κB activation was visualized by EMSA
The inhibitory action of CH11 on NF-κB activation by TNF-α (Figure 1c, lane 2) was completely reversed by preincubation with the pan-caspase inhibitor z-VAD (lane 6), which had no effect by itself (lane 5). These results strongly suggest that caspases participate in downregulation of NF-κB during engagement of the Fas receptor.
Caspase activation induces NEMO and IKK1 cleavage
We next investigated where caspases could act in the NF-κB pathway. After CH11 engagement, we analyzed by immunoblotting the fate of IKK1, IKK2 and NEMO, which are crucial to NF-κB activation. Incubation with CH11 for 6 h led to a sharp drop in NEMO levels (Figure 2a, lane 2) followed by a complete disappearance of the protein 2 h later (lane 3). Similarly, IKK1 was also degraded, although with slower kinetics. On the contrary, IKK2 levels were not affected by apoptosis induction. The cleavage of NEMO and IKK1 corresponded with proteolysis of the poly ADP-ribose polymerase (PARP), a specific caspase substrate whose cleavage precedes DNA fragmentation. As a control, no differences in cellular hsp60 levels were observed after induction of apoptosis.
IKK1 and NEMO are cleaved by caspases. (a) Western blot analysis of signalsome proteins: Jurkat cells were stimulated with CH11 (100 ng/ml) for 6 and 8 h. After lysis, proteins were separated by SDS-PAGE using 10% polyacrylamide gels and blotted on Immobilon membranes. Proteins were revealed using anti-IKK1, IKK2, NEMO, PARP and hsp60 antibodies. (b) Jurkat cells were incubated with CH11 (100 ng/ml) and harvested at indicated times before Western blot analysis. (c) Jurkat cells were incubated with TNF-α (10 ng/ml) and the IKK2 inhibitor AS602868 (10 μM) for 18 or 24 h. Proteins were revealed by Western blot with indicated antibodies. (d) Jurkat cells were incubated with z-VAD-FMK (50 μM) for 24 h before stimulation with CH11 (100 ng/ml) or staurosporine (1 μM) for 8 h. Cells were lyzed and proteins were blotted and revealed as in (a). (e) Jurkat cells deficient for caspase-8 (top 2 panels) or caspase-9 (bottom 2 panels) were incubated with CH11 (100 ng/ml) or staurosporine (1 μM) for the indicated times. NEMO and hsp60 were detected by Western blot. (f) Jurkat cells were incubated for 24 h with the caspase inhibitors DEVD-CHO (30 μM) before incubation with CH11 (100 ng/ml) for 6 h. NEMO and hsp60 were revealed by Western blot. (g) Jurkat cells were incubated for 8 h with CH11 (100 ng/ml). Proteins were separated on a 12% polyacrylamide gel and revealed with an anti-NEMO antibody. (h) Jurkat cells were stimulated with CH11 (100 ng/ml) for 8 h. Cells were permeabilized and stained with anti-Myc antibody and propidium iodide followed by confocal microscopy
A more precise kinetic assessment of NEMO and PARP cleavages was then conducted (Figure 2b), which showed that PARP was proteolysed as early as 90 min (lane 4) after Fas engagement while NEMO disappeared only after 3 h (lane 7). Hsp60 levels did not vary. Depending on SDS-PAGE conditions (size of gel, % of acrylamide), NEMO appeared as a single band or a doublet.
Blockade of NF-κB activation by a pharmacological inhibitor of IKK2 (AS602868) reveals the apoptotic potential of TNF-α.12 Under these conditions, a disappearance of NEMO was detected by Western blotting after 18 (Figure 2c, lane 2) or 24 h (lane 3). Neither TNF-α (lanes 6 and 7) nor AS602868 alone (lanes 4 and 5) affected NEMO levels. Hsp60 levels remain unchanged (lanes 1–7).
Apart from surface death receptors, a second cell-suicide pathway involves the initiator caspase-9 at the mitochondrial level (intrinsic pathway).24 Stimulation of Jurkat cells with staurosporine (STS) induced a disappearance of NEMO and IKK1 (Figure 2d, lane 5). The effect of STS was stronger than that of CH11 (lane 4) and was less sensitive to a blockade by z-VAD (lane 6) compared to CH11 (lane 3). Interestingly, z-VAD alone increased the cellular levels of both IKK1 and NEMO (lane 2). No change in hsp60 levels could be observed throughout the experiment (lower panel). These results strongly suggest that inhibition of NF-κB could be secondary to the cleavage of NEMO or IKK1 by caspases during mobilization of the extrinsic or the intrinsic apoptotic pathways. Since genetic experiments have demonstrated that TNF-α signaling relies more on NEMO and IKK2 than on IKK1,4, 25 we focused our efforts on defining the action of caspases at the level of NEMO, which is essential for the integrity as well as the function of the IKK complex.5
We next used caspase-8- and caspase-9-deficient Jurkat variants to study their participation in the cleavage (Figure 2e). In Jurkat cells, as expected, caspase-8 appeared mandatory for CH11- (lanes 2–4) but not for STS-induced cleavage of NEMO (lanes 5–7). In the absence of caspase-9, neither Fas engagement nor STS stimulation resulted in proteolysis of NEMO. In Jurkat cells (type II), apoptotic events rely on expression of caspase-926.
Preincubation of Jurkat cells with the caspase-3, -6, -7 inhibitor DEVD-CHO almost completely blocked CH11-induced NEMO cleavage (Figure 2f, lane 4 compared to lane 3). The inhibitor alone had no effect on NEMO levels (lane 2). In that experiment, STS induced a weak decrease in NEMO (lane 5) that was slightly, but not completely, prevented by DEVD-CHO (lane 6). No change in hsp60 levels could be observed (lower panel).
We have been unable to induce cleavage of recombinant or in vitro-translated NEMO by either recombinant purified caspase-3, -6, -7, or apoptotic Jurkat extracts that were fully capable of cleaving the tyrosine kinases fyn and lyn (not shown), two known in vivo caspases substrates.21 As shown in Figure 2g, treatment of Jurkat cells with CH11 for 8 h led to the disappearance of full-length NEMO (48 kDa) and to the simultaneous rise of a 41 kDa protein recognized by anti-NEMO antibodies (lane 2). We have had difficulties in regularly observing this band, suggesting that it could be unstable or in a disturbed conformational state. Incubation with various protease inhibitors (E64, ALLN, MG132, o-phenanthroline) did not stabilize this fragment (not shown).
Confocal experiments were then performed to study the behavior of NEMO after caspase activation. Transfected Jurkat cells were fixed and stained with either propidium iodide or anti-Myc antibodies and a secondary fluorescent antibody (Figure 2h). In unstimulated cells, NEMO signal was located both in the cytosol and at the plasma membrane. After apoptosis induction by CH11 for 8 h, which was evidenced by nuclear condensation of the chromatin (right lower panel), the signal was more diffuse and weaker. In the same field, a cell that did not undergo apoptosis was used as a control.
This result suggests that after cleavage, NEMO is not submitted to relocalization but rather becomes less stable.
Identification of the caspase cleavage site on NEMO
Although the NEMO protein was completely cleaved after induction of apoptosis, we had difficulties in detecting the corresponding proteolysed form of NEMO, which made identification of the cleavage site(s) difficult. Five sites that could be used by caspases27 (Asp: 63, 113, 242, 262, 355) were individually mutated by replacing the aspartic acid by a glutamic acid. The resulting cDNAs coding for myc-tagged versions of NEMO were then transfected in Cos7 cells. The fate of NEMO was assessed by Western blotting with anti-Myc antibodies. Apoptosis was induced by either co-transfection of an active caspase-8 cDNA (Figure 3a) or cell stimulation with STS (Figure 3a, lane 5; Figure 3b). Expression of caspase-8 as well as STS stimulation led to the disappearance of the wild-type form of NEMO (Figure 3a, lanes 3 and 5, respectively), which could be prevented by preincubation with z-VAD (lane 4) suggesting that this experimental model mimics the situation observed in Jurkat cells. Mutation of D355 completely prevented the cleavage of NEMO induced by caspase-8 (Figure 3a, lane 15) or by STS (Figure 3b, lane 13). In contrast, mutation of D63, -113, -242 and -262 did not interfere with the disappearance of NEMO (Figure 3a, lanes 6–7, 8–9, 10–11, 12–13 and Figure 3b, lanes 4–5, 6–7, 8–9, 10–11, respectively). As a control, no variations in hsp60 levels could be observed in apoptotic cells. Jurkat cells were then transiently transfected with wt or D355E forms of myc-tagged NEMO (Figure 3c). Fas engagement led to almost complete cleavage of wt NEMO after 6 h (lane 2) while at the same time point, the D355E mutant was resistant (lane 6). After 18 h, levels of D355E mutant declined, likely as a degradation secondary to apoptosis.
NEMO is cleaved after aspartate 355. (a) Cos7 cells were transfected with the different Myc-NEMO cDNA mutants and active caspase-8 cDNA. Forty-eight hours after transfection, cells were incubated with z-VAD-FMK (50 μM) or with staurosporine (1 μM) for 18 h. NEMO was revealed by Western blot using anti-Myc antibody. (b) Cos7 cells were transfected with the different Myc-NEMO cDNA mutants. Forty-eight hours after transfection, cells were stimulated with staurosporine (1 μM) for 18 h. Proteins were revealed by Western blot as in (a). (c) Jurkat cells were electroporated with Myc-NEMO WT or D355E cDNAs. Forty-eight hours after tranfection, cells were incubated with CH11 (100 ng/ml) for 6, 8 or 18 h. NEMO was revealed as in (a)
These data demonstrate that caspase(s) cleaved NEMO after Asp355 (RIED) resulting in the removal of the last C-terminal 64 amino acids. It is not clear if the caspase-8-induced cleavage is direct or mediated after sequential activation of downstream effector caspases. Cleavage after Asp355 is consistent with the observation of a 41 kDa shorter form of NEMO (instead of 48 kDa). The 41 kDa product of NEMO could be visualized only faintly in Jurkat cells (Figure 2g).
Apoptosis interrupts induction of NF-κB-dependent antiapoptotic genes
Expression of NF-κB target genes involved in antiapoptotic functions was investigated through an RNase protection assay (RPA) analysis. We observed that a 4-h stimulation of Jurkat cells with PMA led to an increase in mRNA for the TNFR adaptors TRAF1, TRAF3 and TRAF4, as well as for the caspase inhibitors c-IAP-1 and c-IAP-2 (Figure 4). Interestingly, induction of apoptosis by CH11 stimulation, a condition that blocks NF-κB activation (Figure 1a), completely prevented expression of these pro-survival genes by PMA (Figure 4).
CH11 inhibits transcription of NF-κB-dependent genes induced by PMA. Jurkat cells were stimulated with CH11 (100 ng/ml) for 4 h before stimulation with PMA (10 ng/ml) for 4 h. Expression of RNA was measured by RNase protection assay using the commercial multiprobe templates: hAPO5 and hAPO2c (Pharmingen, San Diego, CA, USA). Expression of each mRNA was quantified on a Phosphoimager and normalized to the expression level of the two housekeeping genes: L32 and GAPDH
Functional consequences of NEMO cleavage: impairment of NF-κB activation
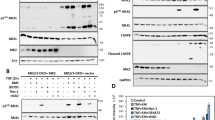
The amino-acid sequence of NEMO reveals two potential coiled-coil motifs as well as a leucine zipper28 and a zinc finger29 (Figure 5a). Mutagenesis experiments30 have shown that the N-terminal coiled-coil domain of NEMO interacts with the IKK kinases; the central coiled-coil region participates in trimerization of the molecule,29 while the C-terminal portion is involved in receiving upstream signals.31, 32 Removal of the last 64 amino acids by caspases results in elimination of the zinc finger (390–412), implying that truncated NEMO could no longer respond to upstream influences. To test this hypothesis, we transfected JM4.5.2 NEMO-deficient Jurkat mutants with cDNAs coding for wt, D355E and del355 mutants. The three different forms of NEMO appeared to be expressed at similar levels, as determined by SDS-PAGE and Western blotting (Figure 5b). As described previously, the absence of NEMO in JM4.5.2 Jurkat variants impaired the ability of TNF-α to activate a transfected κB-luciferase reporter gene (Figure 5c). Transfection of wt and D355E cDNAs restored TNF-α-induced NF-κB activation while expression of the del355 truncated form of NEMO failed to rescue TNF-α signaling.
The cleaved form of NEMO cannot mediate NF-κB activation and confer resistance to TNF-α-induced apoptosis. (a) Schematic representation of the cleaved form of the NEMO protein. κB luciferase assay: JM4.5.2 NEMO-deficient cells were transfected with a κB luciferase reporter gene and pcDNA3-Myc, Myc-NEMO-WT, Myc-NEMO-D355E or Myc-NEMO-del355 plasmids. Forty-eight hours after transfection, cells were stimulated with TNF-α (1 ng/ml) for 18 h and lyzed in 100 μl of lysis buffer. (b) Twenty microliters of total protein extracts were analyzed by Western blot using anti-Myc antibodies. (c) Twenty microliters of total protein extracts were used to measure luciferase activity. Annexin-V analysis of JM4.5.2 NEMO-deficient cell line: JM4.5.2 cells were electroporated with pcDNA-Myc, Myc-NEMO-WT, D355E or del355E and EGFP plasmids. Forty-eight hours after transfection, cells were stimulated with TNF-α (10 ng/ml) (d) or with CH11 (10 ng/ml) (e) for 8 h. Cells were harvested and apoptosis was quantified using annexin-V-PE staining. Shown is a representative of three independent experiments performed in triplicate. Results represent percentages of apoptosis quantified as follows: (TNF-induced apoptosis−apoptosis in medium) × 100/(100−apoptosis in medium) (d) or total percentage of annexin-V-positive cells (e). (f) Twenty micrograms of total protein extract were analyzed by Western blot analysis using anti-Myc antibodies
NF-κB essential modulator cDNAs were then co-transfected with a reporter plasmid coding for EGFP. After cell stimulation and annexin-V-FITC staining, apoptosis of EGFP-positive cells was recorded by FACS.
As a control (Figure 5d), it is shown that TNF-α had no apoptotic effect in normal Jurkat cells (8.05±1.5 vs 5.0±0.01). In contrast, TNF-α stimulation of the JM4.5.2 transfected with empty pcDNA plasmid resulted in a strong annexin-V staining (75.2±1.1 vs 5.1±0.2). A similar response was observed in cells complemented with the del355 form of NEMO (79.4±2.5). By sharp contrast, expression of the D355E NEMO mutant exerted a 3.6-fold protection toward apoptosis that was 2-fold greater than that of wt NEMO (1.7-fold, 43.1±0.5).
In contrast, engagement of Fas by CH11 induced similar responses between pcDNA, WT- or D355E-transfected JM4.5.2 cells (average 83 vs 17%) (Figure 5e).
These results demonstrate that the caspase-generated, D355-deleted form of NEMO is unable to mediate NF-κB signaling, and as a consequence, unable to protect from TNF-induced apoptosis. Moreover, cells expressing a non-cleavable form of NEMO (D355E) are less susceptible to TNF but not CH11-induced apoptosis.
Discussion
Survival and death pathways are in essence interconnected. Cells have to integrate these opposite influences to produce an accurate effector response. NF-κB activation is an important element in the cellular decision to tilt the balance toward survival or death.33 We show here that IKK1 and NEMO, two components of the IKK complex that transmit NF-κB activation signals are cleaved by cellular caspases during the onset of apoptosis in the human leukemic cell line Jurkat. Since NEMO, but not IKK1, is required for TNF-α signaling and engagement of pro-survival actions, we studied the physiological relevance of its cleavage.
While it remains to be determined which caspase targets NEMO, the proteolytic reaction occurs after aspartate 355 to generate a C-terminal truncated form of the protein lacking the last 64 amino acids. This reaction is observed after triggering the Fas death receptor by an agonist antibody, or after mobilization of the mitochondrial pathway by STS. Cleavage was also observed when TNF-α was combined with an IKK2 inhibitor to block NF-κB activation, a condition that is known to reveal the apoptotic potential of the cytokine.12 Our failure to detect cleavage of recombinant- or in vitro-translated-NEMO by recombinant caspase-3, -6, -7 suggests that this reaction may only occur when NEMO is in its native conformation and/or cellular environment.
The C-terminal region of NEMO contains a zinc-finger domain (390–412) integrating upstream signals to NF-κB activation.31, 32 As expected, we observed that the C-terminal-truncated form of NEMO failed to reconstitute NF-κB signaling, suggesting that the reduction in inducible NF-κB DNA binding is likely to result from perturbation of the signaling functions of the IKK complex after NEMO cleavage. The absence of IKK1 is believed to have little-to-no effect on NF-κB activation by TNF-α, as suggested by results from gene inactivation experiments.
Interestingly, the gene coding for NEMO carries several mutations in Incontinentia Pigmenti (IP) patients.34 These mutations result in truncations or additions of unrelated amino acids that modify the C-terminal part of the protein and perturb its ability to transmit incoming signals to IKK1/IKK2. Therefore, cells from IP patients are defective for both NF-κB activation and protection from apoptotic stress.34 Removal or perturbation of this domain is thus likely to have profound consequences on NF-κB activation and cell survival.
We observed here that engagement of the Fas death receptor prevented PMA-induced NF-κB activation and expression of its target genes coding for TRAF1, TRAF3, TRAF4, c-IAP-1 and c-IAP-2. TRAF proteins that are adaptors to the TNF-α receptor have been proposed to amplify NF-κB activation and to enhance its antiapoptotic effects.35 c-IAP proteins act by direct inhibition of caspases, either by steric inhibition or by inducing their degradation.36 The important decrease in expression of these genes is likely to result in a strong decrease in antiapoptotic influences from NF-κB.
In addition, we show that cells expressing a non-cleavable form of NEMO appeared more resistant to cell death induced by TNF-α suggesting that the cleavage of NEMO could represent an amplification loop for apoptosis (see model on Figure 6). The sensitivity of these cells to CH11 did not change as Fas engagement has no effect on NF-κB activation in our model and conditions. Proteins in the NF-κB pathway are preferential targets for caspases. Tang et al.37 described a caspase-3-dependent cleavage of IKK2 that we did not detect in our experimental conditions. In contrast, we observed that IKK1 is degraded during Jurkat apoptosis. The p65/RelA subunit of NF-κB was found to be proteolysed by caspases to generate a dominant negative protein with intact DNA-binding activity but impaired ability to activate transcription.23 Finally, caspases could convert IκB-α into a super repressor of NF-κB activation.38
It is more likely that there is some kind of specificity in these cleavage reactions in vivo. Crucial parameters to select the substrates could be the nature of the death signal, the identity of the caspase, the strength of caspase activation and the cellular context. This would permit differential activity in the NF-κB pathway to block or modulate the levels of NF-κB antiapoptotic actions (cleavage of IKK2 or NEMO) or to regulate the respective engagement of the classical vs the alternative NF-κB activation pathways (cleavage of IKK1 vs NEMO/IKK2).
Another interesting possibility is that under certain conditions, caspases can be activated without leading to apoptosis, for instance during T-cell activation, cell-cycle regulation39 or erythroid differentiation.40 Modulating NF-κB activation through NEMO cleavage could be a means to influence and orientate these processes.
Our results highlight the caspase-dependent cleavage of NEMO as a new post-translational modification to modulate either the strength or the nature of NF-κB activation.
Materials and Methods
Cell culture, reagents and antibodies
Jurkat and its mutant derivatives caspase-8-deficient,41 JMR caspase-9-deficient26 and JM4.5.2 NEMO-deficient,42 were maintained in RPMI-1640 medium containing 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1 mM pyruvate and 5% fetal calf serum. Cos7 cells were maintained in DMEM medium containing 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1 mM pyruvate and 10% fetal bovine serum. IKK1, IKK2 and hsp60 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA), PARP antibody from Cell Signaling Technology (Beverly, MA, USA) and Myc antibody was a kind gift of Dr. JF Tanti (INSERM U568, Nice, France). STS, PMA and regular chemicals were from Sigma-Aldrich (L'isle d'Abeau, France). Anti-Fas IgM (CH11) was obtained from Upstate Biotechnology (Lake Placid, NY, USA), Z-VAD-FMK from Biovision Inc. (Clinisciences, Montrouge, France), TNF-α from PeproTech (Rocky Hill, NJ, USA), AS602868 from Serono International (Genève, Switzerland) and luciferin was from Promega (Madison, WI, USA).
Electrophoretic mobility shift assays
Cellular extracts and mobility shift assays were performed as described.12
Western blot analysis
Total cell extracts were separated by SDS-PAGE on polyacrylamide gels and blotted on Immobilon membranes.12 Binding of primary specific antibodies was revealed with a secondary-peroxidase-conjugated-antibody (DakoCytomation, Glostrup, Denmark) followed by ECL detection (Amersham Pharmacia, Saclay, France).
Confocal microscopy
Stimulated cells (106) were washed with PBS and fixed with 1% formaldehyde for 15 min at room temperature. Cells were permeabilized with digitonine (10 μg/ml) for 7 min at room temperature and incubated with anti-NEMO antibody diluted in RPMI medium containing 10% FBS for 45 min at 4 °C. Cells were then incubated with a FITC-coupled secondary antibody 30 min in the dark and incubated with propidium iodide for 1 min. Cells were then resuspended in 30 μl of mowiol buffer. Signals were analyzed with a TCS-SP confocal microscope (Leica, Heidelberg, Germany) using a 63 × magnification lens. Each picture represents the projection of eight serial confocal optical sections.
Plasmids and mutations
NF-κB essential modulator cDNA was subcloned into a myc-tagged pcDNA3 plasmid. The myc-tagged putative caspase-resistant NEMO mutants were constructed by site-directed mutagenesis using the Quikchange kit (Stratagene). Suspected aspartic acids were mutated into glutamic acids. The myc-tagged short form of NEMO was also obtained by site-directed mutagenesis of the methionine 356 codon into a stop codon. All constructs were confirmed by sequencing.
Cell transfection
Cos7 cells were transfected with 5 μg of Myc-NEMO cDNA and 5 μg of active caspase-8 cDNA by DEAE-dextran method as described previously.43 Forty-eight hours after transfection, cells were harvested and lysed for further analysis. JM4.5.2 and Jurkat cells were electroporated (320 V, 960 μF) with 8 (JM4.5.2) or 10 μg (Jurkat) of the different NEMO constructs and 2 μg of luciferase plasmid or EGFP plasmid.
Luciferase assays
JM4.5.2 NEMO-deficient cells were electroporated with 8 μg of the different NEMO constructs and 2 μg of a luciferase reporter gene controlled by a minimal thymidine kinase promoter with three reiterated κB sites (κBx3 thymidine kinase luc). Forty-eight hours after transfection, cells were stimulated for 18 h as indicated and lyzed in 100 μl of commercial buffer (Promega, Madison, WI, USA). Soluble extracts were assayed for luciferase activity on a Centro LB960 luminometer (Berthold,Thoiry, France).
RNase protection assay
After stimulation, cells were harvested, and total RNA isolated using the TriPure reagent (Boehringer, Mannheim, Germany). Five micrograms of total RNA were hybridized for 18 h with the 32P-labeled riboprobes (PharMingen San Diego, CA, USA) and digested with RNase. The remaining protected probes were purified, resolved on denaturing polyacrylamide gels and analyzed as described previously.17
Measurement of apoptosis by annexin-V analysis
After stimulation, cells were harvested by centrifugation and resuspended in 100 μl of commercial binding buffer containing annexin-V PE (Biovision, CA, USA). After 15 min incubation in the dark, at room temperature 400 μl of binding buffer was added to the cells and apoptosis analyzed using the FL-2 channel of a FACScan (Becton Dickinson, Cowley, UK).
Abbreviations
- EMSA:
-
electrophoretic mobility shift assay
- IκB:
-
inhibitor of kappa B
- IAP:
-
inhibitor of apoptosis
- IKK:
-
I kappa B kinase
- NEMO:
-
NF-κB essential modulator
- NF-κB:
-
nuclear factor kappa B
- PARP:
-
poly(ADP ribose) polymerase
- PMA:
-
phorbol myristate acetate
- RPA:
-
ribonuclease protection assay
- STS:
-
staurosporine
- TNF-α:
-
tumor necrosis factor
- TRAF:
-
TNF receptor associated factor
References
Karin M, Ben-Neriah Y . Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immmunol 2000; 18: 621–663.
Pahl H . Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999; 18: 6853–6866.
Scheidereit C . IκB kinase complexes: gateways to NF-κB activation and transcription. Oncogene 2006; 25: 6685–6705.
Pomerantz J, Baltimore D . Two pathways to NF-κB. Mol Cell 2002; 10: 693–701.
Yamaoka S, Courtois G, Bessia C, Whiteside S, Weil R, Agou F et al. Complementation cloning of NEMO a component of the IκB kinase complex essential for NF-κB activation. Cell 1998; 93: 1231–1240.
Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D . Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995; 376: 167–170.
Li Q, Van Antwerp D, Mercurio F, Lee K, Verma I . Severe liver degeneration in mice lacking the IκB Kinase 2 gene. Science 1999; 284: 321–325.
Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev 2000; 14: 854–862.
Cheng Q, Lee H, Li Y, Parks T, Cheng G . Upregulation of Bcl-x and Bfl-1 as a potential mechanism of chemoresistance, which can be overcome by NF-κB inhibition. Oncogene 2000; 19: 4936–4940.
Karin M, Lin A . NF-κB at the crossroads of life and death. Nat Immunol 2002; 3: 221–227.
Van Antwerp DJ, Martin SJ, Verma IM, Green DR . Inhibition of TNF-induced apoptosis by NF-kappa B. Trends Cell Biol 1998; 8: 107–111.
Frelin C, Imbert V, Griessinger M, Loubat A, Dreano M, Peyron J-F . AS602868, a pharmacological inhibitor of IKK2, reveals the apoptotic potential of TNF-alpha in Jurkat leukemic T cells. Oncogene 2003; 22: 8187–8194.
Keane M, Rubinstein Y, Cuello M, Ettenberg S, Banerjee P, Nau M et al. Inhibition of NF-kappaB activity enhances TRAIL mediated apoptosis in breast cancer cell lines. Breast Cancer Res Treat 2000; 64: 211–219.
Wang CY, Mayo MW, Baldwin Jr AS . TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 1996; 274: 784–787.
Bottero V, Busuttil V, Loubat A, Magné N, Fischel J, Milano G et al. Activation of Nuclear Factor κB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res 2001; 61: 7785–7791.
Frelin C, Imbert V, Griessinger M, Peyron A, Rochet N, Philip P et al. Targeting NF-κB activation via pharmacological inhibition of IKK2, induced apoptosis of human acute myeloid leukemia cells. Blood 2005; 105: 804–811.
Busuttil V, Bottero V, Frelin C, Imbert V, Ricci J, Auberger P et al. Blocking NF-κB activation in Jurkat leukemic T cells converts the survival agent and tumor promoter PMA into an apoptotic effector. Oncogene 2002; 21: 3213–3224.
Karin M, Cao Y, Greten F, Li Z . NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2: 301–310.
Fischer U, Jänicke R, Schulze-Osthoff K . Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ 2003; 10: 76–100.
Degterev A, Boyce M, Yuan J . A decade of caspases. Oncogene 2003; 22: 8543–8567.
Luciano F, Ricci J, Auberger P . Cleavage of Fyn and Lyn in their N-terminal unique regions during induction of apoptosis: a new mechanism for Src kinase regulation. Oncogene 2001; 20: 4935–4941.
Bertolotto C, Ricci J, Luciano F, Mari B, Chambard J, Auberger P . Cleavage of the serum response factor during death receptor-induced apoptosis results in an inhibition of the c-fos promoter transcriptional activity. J Biol Chem 2000; 275: 37246–37250.
Levkau B, Scatena M, Giachelli C, Ross R, raines E . Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-κB loop. Nat Cell Biol 1999; 1: 227–233.
Desagher S, Martinou M . Mitochondria as the central control point of apoptosis. Trends Cell Biol 2000; 10: 369–377.
Silverman N, Maniatis T . NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev 2001; 15: 2321–2342.
Samraj A, Keil E, Ueffing N, Schulze-Osthoff K, Schmitz I . Loss of caspase-9 provides genetic evidence for the type I/II concept of CD95-mediated apoptosis. J Biol Chem 2006; 40: 29652–29659.
Earnshaw W, Martins L, Kaufmann S . Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999; 68: 383–424.
Rothwarf D, Zandi E, Natoli G, Karin M . IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 1998; 395: 297–300.
Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israël A et al. NEMO trimerizes through its coiled-coil C-terminal domain. J Biol Chem 2002; 277: 17464–17475.
Ye J, Xie X, Tarassishin L, Horwitz M . Regulation of the NF-κB activation pathway by isolated domains of FIP3/IKKγ, a component of the IκB-α kinase complex. J Biol Chem 2000; 275: 9882–9889.
Huang T, Feinberg S, Suryanarayanan S, Miyamoto S . The zinc finger of NEMO is selectively required for NF-κB activation by UV radiation and topoisomerase poisons. Mol Cell Biol 2002; 22: 5813–5825.
Makris C, Roberts J, Karin M . The carboxy-terminal region of IκB kinase γ (IKKγ) is required for full IKK activation. Mol Cell Biol 2002; 22: 6573–6581.
Kucharczack J, Simmons M, Fan Y, Gélinas C . To be, or not to be: NF-κB is the answer-role of Rel/NF-κB in the regulation of apoptosis. Oncogene 2003; 22: 8961–8982.
Courtois G, Smahi A, Israël A . NEMO/IKKγ: linking NF-κB to human disease. Trends Mol Med 2001; 7: 427–430.
Arch RH, Gedrich RW, Thompson CB . Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev 1998; 12: 2821–2830.
Deveraux Q, Reed J . IAP familly proteins-suppressors of apoptosis. Genes Dev 1999; 13: 239–252.
Tang G, Yang J, Minemoto Y, Lin A . Blocking caspase-3 mediated proteolysis of IKKβ suppresses TNFα-induced apoptosis. Mol Cell 2001; 8: 1005–1016.
Reuther J, Baldwin Jr A . Apoptosis promotes a caspase-induced amino-terminal truncation of IκBα as a stable inhibitor of NF-κB. J Biol Chem 1999; 274: 20664–20670.
Los M, Stroh C, Jänicke R, Engels I, Schulze-Osthoff K . Caspases: more than just killers? Trends Immunol 2001; 22: 31–34.
Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F et al. Caspase activation is required for terminal erythroid differentiation. J Exp Med 2001; 193: 247–254.
Juo P, Kuo C, Yuan J, Blenis J . Essential requirement for caspase-8/FLICE in the initiation of the Fas-induced apoptotic cascade. Curr Biol 1998; 8: 1001–1008.
Harhaj E, Good L, Xiao G, Uhlik M, Cvijic M, Rivera-Walsh I et al. Somatic mutagenesis studies of NF-κB signaling in human T cells: evidence for an essential role of IKKγ in NF-κB activation by T-cell costimulatory signals and HTLV-I tax protein. Oncogene 2000; 19: 1448–1456.
Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner BM, Mueller-Dieckmann C et al. Tyrosine phosphorylation of IκB-α activates NF-κB without proteolytic degradation of IκB-α. Cell 1996; 86: 787–798.
Acknowledgements
We appreciate the critical reading of the manuscript by Dr. Robert Herrington (Toronto, Canada). We thank Drs. M Dreano (Merck-Serono International, Geneva, Switzerland), J Blenis (Boston, USA) and JC Chambard (CNRS, Nice, France) for the kind gift of AS602868, caspase-8 deficient Jurkat variants, active caspase-8 cDNA respectively. This work is supported by INSERM and grants from ARC (3392) and la Fondation de France, comité Leucémies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by B Dynlacht
Rights and permissions
About this article
Cite this article
Frelin, C., Imbert, V., Bottero, V. et al. Inhibition of the NF-κB survival pathway via caspase-dependent cleavage of the IKK complex scaffold protein and NF-κB essential modulator NEMO. Cell Death Differ 15, 152–160 (2008). https://doi.org/10.1038/sj.cdd.4402240
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402240
Keywords
This article is cited by
-
Mitochondria and cell death-associated inflammation
Cell Death & Differentiation (2023)
-
Apoptotic caspase inhibits innate immune signaling by cleaving NF-κBs in both Mammals and Flies
Cell Death & Disease (2022)
-
Crosstalk in NF-κB signaling pathways
Nature Immunology (2011)
-
Heat stress triggers apoptosis by impairing NF-κB survival signaling in malignant B cells
Leukemia (2010)