Abstract
The HIV-1 encoded apoptogenic protein Vpr induces mitochondrial membrane permeabilization (MMP) via interactions with the voltage-dependent anion channel (VDAC) and the adenine nucleotide translocator (ANT). We have designed a peptide, TEAM-VP, composed of two functional domains, one a tumor blood vessel RGD-like ‘homing’ motif and the other an MMP-inducing sequence derived from Vpr. When added to isolated mitochondria, TEAM-VP interacts with ANT and VDAC, reduces oxygen consumption and overcomes Bcl-2 protection to cause inner and outer MMP. TEAM-VP specifically recognizes cell-surface expressed αVβ3 integrins, internalizes, temporarily localizes to lysosomes and progressively co-distributes with the mitochondrial compartment with no sign of lysosomal membrane permeabilization. Finally TEAM-VP reaches mitochondria of angiogenic endothelial cells to induce mitochondrial fission, dissipation of the mitochondrial transmembrane potential (ΔΨm), cytochrome c release and apoptosis hallmarks. Hence, this chimeric peptide constitutes the first example of a virus-derived mitochondriotoxic compound as a candidate to kill selectively tumor neo-endothelia.
Similar content being viewed by others
Main
Within the integrin family, αVβ3 receptors are considered to be an important target for anticancer therapy being upregulated in certain invasive tumors.1 αVβ3 recognizes a specific consensus sequence, an RGD motif, which is present in a number of extracellular matrix proteins like vitronectin, fibronectin, fibrinogen and thrombospondin.2 RGD-containing peptides have been shown to reduce angiogenesis induced by cytokines and to mediate apoptosis in endothelial cells.3 It appears that RGD peptides that are constrained in a cyclic conformation, show an increased affinity for binding to integrins.4 One example is the cyclic RGD-4C peptide, a potent binder of α5β1, αVβ3 and αVβ5 used to selectively deliver doxorubicin to tumor blood vessels5 and to internalize the amphipatic peptide (KLAKLAK)2 in endothelial cells resulting in cellular apoptosis.6
A number of viral genomes have evolved to hijack apoptotic cell death processes. Various virus families encode proteins that target mitochondrial membranes to trigger cell death. Such proteins include HTLV P13II, Influenza virus PB1-F2, HCV P7, HPV E1Ê4, Hbx and HIV-1 Vpr.7, 8 These proteins share a structural criterion in that they contain at least one positively charged amphipathic alpha-helix. Vpr encodes a 96-amino acid, 14 kDa protein that performs multiple functions, including the induction of apoptosis.9 By association with the mitochondrial membrane, Vpr triggers a typical mitochondrial pathway of apoptosis characterized by an early loss of the inner transmembrane potential (ΔΨm), the release of cytochrome c, the loss of respiratory activity and activation of various caspases including caspases 9 and 3.9, 10, 11 Genetic evidence in yeast, cooperative channel formation in planar lipid bilayer, affinity purification and plasmon resonance studies showed that the C-terminal Vpr moiety (Vpr52-96) binds to the adenine nucleotide translocator (ANT) and may interact with the voltage-dependent anion channel (VDAC).10, 11, 12 We have suggested that these interactions may explain how Vpr (and derived C-terminal peptides) induces permeability transition.10, 11 Indeed, the permeability transition pore (PTP) is assumed to be due to the formation of a dynamic multiprotein complex at outer and inner mitochondrial membrane contact sites.13 The exact composition of the PTP is still unknown but appears to contain VDAC in the outer membrane and is regulated by ANT in the inner membrane, and cyclophilin D (the target for cyclosporin A, CsA), located in the matrix.13, 14, 15, 16
In an attempt to generate new angio-toxic molecules as candidates for cancer drug development, we designed chimeric peptides composed of αVβ3-binding peptides and apoptogenic sequences derived from the mitochondrio-active portion of Vpr. One of these peptides, called TEAM-VP (for Targeted to Endothelial Apoptogenic Mitochondrio-active Vpr-derived Peptide), is composed of the cysteine-cyclised peptide sequence GGCRGDMFGC4 and a Vpr67-82 sequence derivative. We show here that short Vpr-derived peptides induce both inner and outer mitochondrial membrane permeabilization, and that the cyclic GGCRGDMFGC peptide specifically binds and internalizes in αVβ3-expressing cells. Moreover, a detailed study of TEAM-VP's cellular effects, reveals that it internalizes specifically in αVβ3 expressing cells through its cyclic RGD motif, leaves the endo-lysosomal compartment to reach mitochondria and induces an intrinsic-like pathway of apoptosis.
Results
Design of chimeric structures to selectively kill αVβ3-expressing cells
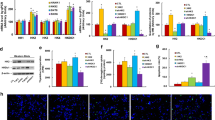
A systematic screen on isolated mitochondria of synthetic Vpr-derived peptides having a potent MMP inducing activity (ED50 <1 μM) led us to optimize a 16-mer peptide (termed VP) LLFIHFRIGSRHSRIG (Vpr67-82[C76S]), as it is the shortest strongly active, low oxydizable sequence (Figure 1a).11 First we checked the mitochondriotoxicity of this mutated peptide Vpr67-82[C76S]. When added to purified mitochondria, VP induces inner and outer MMP hallmarks including matrix swelling (Figure 1a), ΔΨm dissipation (Figure 1b), mitochondrial volume increase (Figure 1c) and extramitochondrial release of cytochrome c (Figure 1d). Swelling is also induced with DVP, in which the amino-acids are in absolute configuration D (Figure 1a). Replacement of critical arginine (R) residues (R73A and R80A) in VP greatly reduces MMP (mVP; Figure 1a,b,d). VP-induced MMP is reduced by pre-treatment of mitochondria with the broad spectrum anion channel inhibitor 4,4′-diisothiocyanastilbene-2,2′-difulfonic acid disodium salt (DIDS) but not with the PTP inhibitor (closer) cyclosporin A (Figure 1a). This latter observation may appear surprising since, Vpr-derived peptides were previously found to induce PTP opening through a CsA-sensitive pathway.10, 11 Modifications in the VP sequence (comparatively to Vpr) such as length and/or single amino-acid changes may explain part of such functional variations (see discussion). Interestingly, in contrast to Vpr52-96, the shorter peptide VP has very low toxicity when added to various cell cultures (not shown). This appears to be due (at least in part) to lower plasma membrane transducing capacities. When VP is linked to the well defined Tat[49–57] peptide transduction domain, the resulting peptide has a high capacity to transduce plasma membranes and efficiently induces cell killing of various cell types including HUVECs (Figure 2a). Exploring the design of a Vpr-derived peptide with a selective antitumor effect, we constructed bi-functional peptides containing different RGD-derived cyclic peptides (Figure 2a). When linked to synthetic cyclic GGCRGDMFGC (cycRGD) targeting moiety, VP becomes TEAM-VP a potent killer of HUVECs (Figure 2). Systematic dose–response studies reveal that, in the context of this cyclic RGD targeting system, VP is potent (LD50 at 24 h for TEAM-VP: 15 μM) to kill HUVECs whereas cotreatment with the VP peptide or an equimolar mixture of VP plus cycRGD has low cytotoxicity (Figure 2a,b). Within the same chimeric RGD-containing constructs, synthetic Bcl-2 homology domains BH3 peptides (from Bid or Bax) do not induce HUVEC cell death. Other established permeability transition facilitators such as the 14-amino acid wasp venom-derived amphipathic peptide mastoparan,17 have poor efficacy to kill HUVEC when combined with cycRGD (Figure 2a,b). As expected killing efficiency of TEAM-VP increases with time in prolonged HUVEC cultures (viability loss at 72 h >80% at 15 μM; data not shown). In order to enhance the apoptogenic properties of TEAM-VP, we also synthetized a peptide containing non-natural amino acids with absolute configuration D in the 16-mer (VP) portion of TEAM-VP (Figure 2a,b). As expected, the resulting TEAM-DVP has a stronger cytotoxicity on HUVECs (DL50=6.8±1.5 μM).
Vpr67-82[C76S] effects on isolated mitochondria. (a) Vpr67-82[C76S]-induced swelling. Mouse liver mitochondria were exposed to 30 μM Ca2+, 0.5 μM Vpr67-82[C76S] (called VP), 0.5 μM DVpr67-82[C76S] (called DVP), or a mutated and inactive form of Vpr67-82 [C76S; R73,80A] (mVP), in the presence or absence of DIDS (2-16 μM) or cyclosporin A (CsA; 10 μM). Mitochondrial swelling (measured as 90° light scattering at 545 nm) was monitored continuously. Representative curves are shown in the left panel. Quantitation of the frequency of swollen mitochondria (mean±S.D., n=3) is shown in the right panel. The percentage of swelling is calculated as described in the Materials and Methods section. (b) VP-induced ΔΨm dissipation. Isolated mitochondria were treated as in A (VP with or without 16 μM DIDS) or with carbonyl cyanide meta-chlorophenylhydrazone (mClCCP; 20 μM). ΔΨm dissipation (measured by JC-1 orange fluorescence flow cytometry detection)38 was monitored continuously. Representative curves are shown in the left panel. Percentage of ΔΨm low mitochondria (right panel; mean±S.D., n=3) is calculated as the frequency of JC-1 orange low events (collected in the FL-2 channel; 585±21 nm. (c) Ultrastructural effects of VP on isolated mitochondria. Electron micrographs were obtained after incubation of isolated mitochondria with or without VP (1 μM) with or without a 5-min preincubation with DIDS (5 μM). The percentage of swollen mitochondria was <5% in the control, >80% 15 min after VP addition and <15% after VP+DIDS treatment (n=3). Scale bars 1 μm. (d) VP-induced mitochondrial release of cytochrome c. Mitochondrial supernatants treated as in (a) or with Alamethicin (positive control;38 5 μg/ml) were subjected to immunoblot detection of cytochrome c
Cytotoxicity of targeted peptides on endothelial cells. (a) List, amino-acid sequence and HUVEC killing capacity of various targeted peptides and control peptides. Peptide sequences are indicated using the one letter code. For clarity, functionnal part of each peptide (targeting sequence, linker sequence and mitochondrio-active sequence) is spaced by a dash. Asterisks on the left of cysteine residues stand for disulfide bonds. Various motifs were used: Tat(49-57), Vpr67-82[C76S] (=VP), DVpr67-82[C76S] (=DVP), hBaxBH3(57-72[C62S]), hBidBH3(84-99) and Mastoparan(1–14). All peptides were tested on HUVEC cells and analyzed for their ability to induce cell death. The indicated ED50 is based on plasma membrane permeability evaluation (7-AAD incorporation) measured by flow cytometry after a 24 h treatment with peptides. (b) Cytocidal effects of TEAM-VP on primary human endothelial cells (HUVEC). Cells were incubated for 24 h with 5–30 μM of CycRGD, VP, CycRGD+VP (equimolar mix), TEAM-VP or TEAM-DVP peptides, stained with 7-AAD and analyzed by flow cytometry (mean±S.D., n=4)
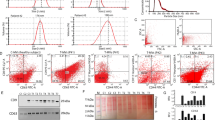
The cycRGD motif contained in TEAM-VP was analyzed for its ability to recognize αVβ3 integrins. Cytofluorometric assessment using FITC-labeled RGD peptides reveal that cycRGD (but not linear RGD nor mutated cycRAD) binds to HUVECs' plasma membrane (Figure 3a). This binding is specific since an excess of unlabeled cycRGD competes with FITC-cycRGD (Figure 3b,c). In addition, the αVβ3 peptidic ligand GRGDS (but not the GRGES control or cycRAD) reduces FITC-cycRGD binding efficiency (Figure 3c). Investigation of a panel of primary and transformed cells shows a strong correlation between αVβ3 (but not αVβ5) expression, cell surface binding and TEAM-VP-induced cell death (Figure 3d). At 15 min post-treatment FITC-cycRGD accumulates at the level of plasma membrane (sometimes near to the fluid phase markers 40S Dextran-TRITC beads18 (insert in Figure 3e) and after 3–5 h intracellular distribution can be objectived (Figure 3e, lower left micrograph). This internalization appears specific since FITC-cycRGD does not enter into αVβ3 negative HeLa cells and FITC-cycRAD does not enter into HUVECs (Figure 3e). A similar situation occurs with TEAM-VP, which enters into cells with a kinetic similar to 40S Dextran beads (Figure 3f, upper micrograph). To investigate if TEAM-VP internalization was energy-dependent, we used the combination of azide and deoxyglucose (to deplete cells in ATP by glycolysis inhibition and block oxidative phosphorylation18, 19 (Figure 3f, lower micrograph) or nystatin (a cholesterol sequestrator reported to block caveolae-mediated endocytosis;20 not shown). These treatments fully block TEAM-VP (and Dextran beads) internalization suggesting an endocytosis-like process (in correlation with confocal analysis of TEAM-VP localization in late endosomal/lysosomal compartments; Figure 4a). Hence, TEAM-VP is a potent peptidic cell killer that specifically recognizes and kills αVβ3-expressing cells after cell entry through an endocytosis-like pathway.
CycRGD recognition by the αVβ3 integrins. (a) Binding of the CycRGD peptide on HUVECs. Cells were incubated for 45 min at RT with the following FITC-labeled peptides: linear RGD, CycRAD or CycRGD at indicated concentrations. Histogram shows fluorescence intensity detected by flow cytometry (one representative experiment). Insert shows primary histogram data with the corresponding color code for each peptide (relative fluorescence intensity, RFI). (b) Specific binding of CycRGD peptide to HUVEC cell surface. Cells were preincubated or not for 30 min at 4°C with 200 μM of unlabeled CycRGD peptide before addition of 10 μM FITC-CycRGD for 45 min and analyzed by flow cytometry. Relative fluorescence intensity (RFI) of FITC-CycRGD bound to HUVECs is represented (one representative experiment). (c) Peptides competition for integrins. HUVEC cells were preincubated or not for 30 min at RT with 25 μM of CycRGD, CycRAD, GRGDS or GRGES peptides before addition of FITC-CycRGD (0.5 μM). Percentage of fluorescent positive cells was measured by flow cytometry (mean±SD, n=3). (d) Comparative analysis of αVβ3 expression, cycRGD binding, TEAM-VP binding, and TEAM-VP-induced cell death on various cell types (HUVEC, HMVECd, A375M, MCF-7, MDA-MB-231, HeLa, HT-29, Jurkat, CEM and fresh peripheral blood mononuclear cells (PBMC)). Cells were labeled with PE-conjugated antibodies directed against the αVβ3 and αVβ5 integrins and analyzed by flow cytometry. Binding of the FITC-CycRGD peptide was analyzed by flow cytometry after cell treatment as in A with 4 μM FITC-CycRGD; the indicated percentages are relative to the binding of FITC-CycRAD (negative control) to each cell type. Binding of TEAM-VP was analyzed by flow cytometry after cells treatment with 4 μM of biotinylated-TEAM-VP+1 μg/ml of streptavidin-FITC for 1 h at RT; the indicated percentages are relative to cells incubated with streptavidin-FITC alone (negative control) (ND: not determined). Cell death induction by TEAM-VP are indicated for each cell types; percentages of 7-AAD positive cells when treated as in Figure 2 with 30 μM TEAM-VP are indicated. (e) Entry of the CycRGD peptide in HUVEC cells. HUVEC or HeLa cells were treated simultaneously with 20 μM of FITC-CycRGD or FITC-CycRAD (green fluorescence) and 1 mg/ml Dextran-TRITC beads (red fluorescence) for 15 min or 5 h at 37°C and stained with the DNA intercalating dye Hoechst 33342 (blue fluorescence) followed by fluorescence microscopy observation. Insertion in the picture at 15 min incubation shows localization of the CycRGD peptide and Dextran beads at the plasma membrane of a HUVEC cell (fluorescence+phase-contrast). Scale bars, 10 μm. (f) Entry of TEAM-VP in HUVEC cells. Cells were co-treated with 2.5 μM of biotinylated-TEAM-VP and 1 mg/ml Dextran-TRITC beads (red fluorescence) for 5 h at 37°C in the presence or absence of 2-deoxyglucose and NaN3, (endocytosis inhibition) fixed and stained with a streptavidin-FITC conjugate (green fluorescence) and observed by fluorescence microscopy after Hoechst staining. Note the clear overlap between the green and red fluorescence (yellow), indicating a cytoplasmic codistribution of TEAM-VP with the fluid-phase endocytosis marker (Dextran beads) (upper panel) and endocytosis inhibition of both TEAM-VP and Dextran beads (lower panel). Micrographs are representative for >90% of the cells (n=4). Scale bars, 10 μm
Subcellular localization of TEAM-VP in HUVECs. Micrographs are confocal images of HUVEC cells treated or not with 2.5 μM of biotinylated-TEAM-VP for 8, 24 or 32 h. After fixation, cells were labeled with (a) streptavidin-Texas Red (TEAM-VP, red fluorescence) and anti-Lamp2-FITC (lysosomes, green fluorescence) or (b) streptavidin-FITC (TEAM-VP, green fluorescence) and anti-Porin (mitochondria, revealed Alexa fluor® 546, red fluorescence) or (c) streptavidin-Texas Red (TEAM-VP, red fluorescence) and anti-Golgin-97 (Golgi apparatus, FITC, green fluorescence) and analyzed on Axiovert 200 M with a Zeiss LSM 510 confocal microscope. TEAM-VP colocalizing with lysosomes (a) and mitochondria (b) is visualized as yellow fluorescence. Micrographs (a, b, c) are representative for >70% of the cells (n=3). An additional analysis of the codistribution is presented in Supplementary Materials. (d) Left panels represent a deconvolution of pictures showing cells labeled with streptavidin-Texas Red (TEAM-VP, red fluorescence) and anti-Lamp2-FITC (lysosomes, green fluorescence) at 8 h (1) and 24 h (4). Pixels with a very high codistribution are shown in white (middle panels, 2 and 5). A three-dimensional reconstruction of a region of (1) and (4) is shown on the right panels (3 and 6, respectively). Movies corresponding to the right panels are presented in Supplementary Materials. All images are representative confocal microscopy sections. Scale bars, 10 μm
TEAM-VP leaves endo-lysosomal compartment to reach mitochondria
Routing of TEAM-VP in HUVEC cells was carefully studied by confocal microscopy (Figure 4). Combined fluorescent detection of biotinylated-TEAM-VP (with fluorescent streptavidin) and organelle specific antibodies indicates that TEAM-VP codistributes early (at 8 h) with endo-lysosomal compartments (anti-Lamp2 antibody; Figure 4a; green fluorescence) and colocalizes partially (at 24 h) and totally (at 32 h) with mitochondria (anti-porin antibody; Figure 4b; red fluorescence). An estimate of pixels, which highly colocalize in each cell is shown in Supplementary Materials (Figure 1a). No localization of the peptide is seen in other organelles such as the Golgi apparatus (Figure 4c). Deconvoluted confocal images and 3D analysis (Figure 4d) (see animation in Supplementary Materials; Figure 1b) reveal that TEAM-VP is found inside lysosomes at 8 h post-treatment (Figure 4d, upper panels) but is detected outside the lysosomal membrane at 24 h (Figure 4d, lower panels). Co-labeling of HUVECs with vital dyes including LysoTraker® red and MitoTracker® green after TEAM-VP addition rule out the possibility that TEAM-VP might have induced organelle contact or spatial proximity between lysosomes and mitochondria (not shown). Overall, these experiments suggest that TEAM-VP may escape from lysosomes to reach the mitochondrial compartment.
TEAM-VP induces both inner and outer MMP
Similarly to VP, TEAM-VP acts directly on isolated mitochondria to induce MMP hallmarks including matrix swelling, ΔΨm dissipation, mitochondrial volume increase and cytochrome c release (Figure 5a–c,e). TEAM-VP-induced MMP is reduced by DIDS (Figure 5a,c,e), but insensitive to CsA (Figure 5a). Replacement of the RGD motif by RAD in TEAM-VP does not affect its ability to induce MMP (peptide C1; Figure 5b,e) contrary to mutation of critical arginine (R) residues (R73A and R80A) in the VP portion (peptide C2; Figure 5b,e). VP linked to Tat[49–57] has the same effect as TEAM-VP on ΔΨm and cytochrome c release (peptide C3; Figure 5b,e). Targeting sequence is silent as TEAM-VP presents the same effect as VP on isolated mitochondria. The D-versions of VP and TEAM-VP (termed DVP and TEAM-DVP, respectively) confer higher MMP-inducing properties to TEAM-VP (Figure 5a,b). Indeed, the swelling dose 50 (SD50) of TEAM-DVP is 0.07±0.01 μM (six times lower than for TEAM-VP) and the depolarization dose 50 (DD50) is 0.13±0.06 μM (four times lower than for TEAM-VP).
Effects of TEAM-VP on isolated mitochondria. (a) TEAM-VP-induced mitochondrial swelling. Mouse liver mitochondria were incubated with 0.5 μM CycRGD, TEAM-VP or TEAM-DVP peptides in the presence or absence of DIDS (8 μM) or CsA (10 μM). Mitochondrial swelling (measured as 90° light scattering at 545 nm) was monitored continuously. The representative curves obtained are shown. (b) TEAM-VP-induced ΔΨm loss. Isolated mitochondria were incubated with 1 μM of CycRGD, TEAM-VP or CycRAD-VP (C1; Table 1), TEAM-mVP (C2; Table 1) and TAT-VP (C3; Table 1), stained with the ΔΨm-sensitive dye JC-1 and finally subjected to flow cytometry analysis. The representative curves are shown (a. u., arbitrary units). (c) Ultrastructural effects of TEAM-VP on isolated mitochondria. Electron micrographs were obtained after incubation of isolated mitochondria with or without TEAM-VP (0.5 μM) with or without a 5-min preincubation with DIDS (5 μM). Scale bars, 0.75 μm. (d) Bcl-xl and Bcl-2-independency of VP variants. Isolated mitochondria from HeLa cells were incubated with recombinant t-Bid (1 nM), TEAM-VP (5 μM), VP (5 μM), TEAM-dVP (2.5 μM) or dVP (2.5 μM) after a 5 min-pretreatment with recombinant Bcl-xl or Bcl-2 (1 μM). Mitochondrial supernatants (left panels) and mitochondrial pellet (right panels) were subjected to immunodetection of cytochrome c. Note that both Bcl-xl and Bcl-2 strongly reduce t-Bid-induced cytochrome c release. (e) TEAM-VP-induced cytochrome c release. Isolated mitochondria were incubated with 1 μM TEAM-VP in the presence (or not) of DIDS (10 μM), or with control peptides (C1, C2 and C3) or with Alamethicin (5 μg/ml), and mitochondrial supernatants were subjected to immunoblot detection of cytochrome c. (f) Affinity purification of PTP components (VDAC and ANT) by biotinylated-TEAM-VP. Mitochondria were exposed for 30 min at RT to 5 μM of biotinylated-CycRGD (negative control), 5 μM of biotinylated-TEAM-VP or 5 μM of biotinylated-Vpr52-96 (positive control)10, 11 peptides, followed by triton solubilization and purification of proteins interacting with TEAM-VP-biotin-streptavidin-agarose. The whole mitochondrial extract (control) or the streptavidin-recovered proteins were separated by SDS-PAGE followed by immunodetection of Hsp60, VDAC (32 kDa) and ANT (30 kDa; Q18 antibody, Santa Cruz). Western-blot for ANT is constituted by two images from different fields of the same gel. (g) Affinity purification of ANT by biotinylated-TEAM-VP in treated HUVECs. Cells were treated for 24 h with 15 μM of biotinylated-CycRGD, TEAM-VP or C2 peptides. Cell lysates were incubated with streptavidin-agarose for purification of proteins interacting with biotinylated-TEAM-VP. The whole cellular extract (control) or the streptavidin-recovered proteins were separated by SDS–PAGE followed by immunodetection with anti-Hsp60 or a polyclonal anti-hANT-1 antibody. (h) Oxidative properties of isolated mitochondria exposed to TEAM-VP. Oxygen consumption curves after addition of the indicated agents. Numbers along the trace are nanomoles of O2 consumed per minute per milligram of protein. Note that in the presence of 2 μM TEAM-VP oxygen consumption is strongly reduced (>50%) but fully restored by external addition of reduced cytochrome c (4 μM). The inserted histogram shows the percentage of oxygen consumption inhibition with increasing concentrations of TEAM-VP
Further series of experiments point out interesting properties of TEAM-VP and derived peptides. For instance, VP family peptides bypass the mitochondrioprotective effect of Bcl-xl and Bcl-2 antiapoptotic proteins on isolated mitochondria. Indeed addition of recombinant Bcl-xl and Bcl-2 to isolated mitochondria prevents tBid-induced but not TEAM-VP-induced cytochrome c release (Figure 5d). Strikingly, such MMP inducing properties and Bcl-2 independence are enhanced using DVP and TEAM-DVP. We then investigated the hypothesis that TEAM-VP might have the same target as Vpr52-96, namely the PTP.10, 11 It appears that biotinylated TEAM-VP can be used as a tool for affinity-mediated purification of ANT and VDAC from isolated liver mitochondria (Figure 5f). These results suggest that TEAM-VP is recruited to the PTP via a direct, specific interaction with ANT and/or VDAC. To investigate whether TEAM-VP may also interact with ANT when it kills cells, cultured HUVEC were incubated for 24 h with biotinylated TEAM-VP (which is as efficient as nonmodified TEAM-VP in inducing HUVEC's ΔΨm loss and death; not shown), followed by cell lysis, crude extract preparation and purification of biotinylated-TEAM-VP binding proteins on avidin-agarose. This led to the recovery of ANT (but not HSP-60) by immunoblotting (Figure 5g). In addition, when added to mitochondria TEAM-VP reduces oxygen consumption (succinate oxidization) in the presence of ADP, in a dose-dependent manner (Figure 5h). Adding cytochrome c to TEAM-VP-treated mitochondria oxidizing succinate stimulates oxygen uptake indicating that TEAM-VP does not primarily affect respiratory chain but rather induces (outer) MMP. When added to HUVEC cells, TEAM-VP is also able to induce ΔΨm dissipation as observed by fluorescence microscopy and FACS analysis (Figure 6a,b). The Tat[49–57]-VP peptide (C3) has the same effect as TEAM-VP (Figure 6b). As expected, replacement by alanine of two arginines (R73,80A) among the three critical arginines (R73,77,80) only partly reduces TEAM-VP toxicity in cellula.10 In situ detection of cytochrome c reveals a massive release of the protein in the cytoplasm in presence of TEAM-VP at 24 h (Figure 6c). Interestingly, fluorescence microscopy on TEAM-VP-treated cells (Figure 6c,d) reveals dramatic changes in the mitochondrial network with small punctiform organelles resembling fragmented mitochondria.21 This is confirmed by electron microscopy (Figure 6e) showing invaginations of OMM (outer mitochondrial membrane) and mitochondria connected by a narrow neck in cells treated with TEAM-VP or mClCCP, a positive control for fission. Mitochondrial fission appears despite the presence of the pan-caspase inhibitor Z-VAD-fmk (Figure 6d) suggesting that TEAM-VP induces early fragmentation of mitochondria, which precedes caspase activation. In conclusion, TEAM-VP induces major cell death associated changes of mitochondria in αVβ3 positive cells.
Mitochondrial effects of TEAM-VP, in cellula. (a) TEAM-VP-induced ΔΨm dissipation in intact HUVEC cells. Fluorescent micrographs represent HUVEC cells incubated or not with TEAM-VP (15 μM) for 16 h (35% of ΔΨm low cells and stage I nuclei) and 24 h (50% of ΔΨm low cells and 10% of stage II nuclei) and stained with 1 μM ΔΨm-sensitive dye JC-1 (orange fluorescence shows mitochondria with high ΔΨm, green fluorescence shows mitochondria with low ΔΨm) and with Hoechst 33342 (blue fluorescence). Scale bars, 10 μm. (b) Effect of sequence modifications on TEAM-VP-induced ΔΨm dissipation. Cells treated with 15 μM of TEAM-VP, CycRAD-VP (C1), TEAM-mVP (C2) or TAT-VP (C3) were stained with JC-1 as in A and subjected to flow cytometry analysis. Percentage of ΔΨm low cells is calculated as the frequency of JC-1 orange low cells. A representative histogram is shown. (c) TEAM-VP-induced mitochondrial release of cytochrome c. Cells treated or not with TEAM-VP (10 μM; 24 h) were fixed, permeabilized and immunostained with antibodies specific for cytochrome c (revealed FITC, green fluorescence) and the mitochondrial sessile protein VDAC (revealed Alexa fluor® 594, red fluorescence) and observed by fluorescence microscopy. A fluorescent micrograph representative of 20% of the treated cells is shown. Scale bars, 10 μm. (d) TEAM-VP-induced mitochondrial fission. Fluorescent micrographs represent cells treated or not for 24 h with 15 μM TEAM-VP in presence or not of 50 μM Z-VAD-fmk, stained with the ΔΨm-insensitive mitochondrial dye 60 nM MitoTracker™ green and Hoechst, and then subjected to fluorescence microscopy observation. Fluorescent micrographs representative of 30% of the treated cells are shown. Scale bars, 10 μm. (e) Mitochondrial fission observed by thin section electron microscopy. Electron micrographs were obtained after treatment or not of HUVEC cells with 15 μM TEAM-VP (48 h) or with 50 μM mClCCP (2 h) (positive control). Note mitochondria connected by a narrow neck (arrows). Scale bars, 0.5 μm
TEAM-VP-induced cell death presents hallmarks of apoptosis
To ensure that the lysosomal membrane permeabilization process was not at the origin of TEAM-VP-induced cell death, we studied the integrity of lysosomal membranes during treatment with TEAM-VP (Figure 7a). Clearly, TEAM-VP does not induce the release of the lysosomal protease cathepsin B which codistributes with lysosomes, indicating that there is no massive lysosomal membrane permeabilization (LMP) in the presence of TEAM-VP, contrary to the treatment with UV and the quinolone antibiotic norfloxacin which induces translocation of cathepsin B from lysosomes (positive control for LMP).22 Accordingly, massive inhibition of catabolic lysosomal proteases with chloroquine and NH4Cl and specific inhibition of cathepsin B by Z-FA-fmk do not affect TEAM-VP-induced ΔΨm loss and cell death (Figure 7b). We further analyzed various cell death parameters, which allow us to chronologically order apoptotic events induced by TEAM-VP (Figure 8a). ΔΨm dissipation is detected early (16 h) and is not inhibited by the pan-caspase inhibitor Q-VD-OPH. ΔΨm dissipation is followed by phosphatidylserines translocation to the outer leaflet of the plasma membrane (24 h). Hypoploïdy estimated by quantification of the sub-G1-phase cells occurs much later (48 h) and is inhibited by the broad spectrum caspase inhibitors Z-VAD-fmk or Q-VD-OPH (Figure 8a). When devoid of its targeted moiety VP is less efficient than TEAM-VP due to the lack of recognition of αVβ3 integrins. Increased caspase-3 like activity is detected at 24 h in cytosolic extract from HUVECs treated with TEAM-VP compared to untreated cells (Figure 8b). In contrast, no increase in caspase-3 like activity is detected in cytosolic extracts from cells cotreated with Q-VD-OPH and TEAM-VP (not shown). We then investigated in detail the appearance of nuclear apoptosis in the presence of TEAM-VP during dose–response and time–response experiments (Figure 8c). Two nuclear stages can be identified: stage I, associated with peripheral chromatin condensation, and stage II in which nuclei are fragmented (Figure 8c, upper panels).23 Stages I and II appear in a dose- and time-dependent manner and the formation of stage II nuclei is inhibited by the pan-caspase inhibitor Z-VAD-fmk (Figure 8c, lower panels) contrary to stage I formation (and cell death). Hence, TEAM-VP targets mitochondria to induce caspase-dependent and caspase-independent nuclear apoptotic events. We further investigated the implication of the proapoptotic proteins Bax and Bak in the VP-induced cell death by using the HCT-116 Bax (+/−) (control) and Bax/Bak double knockout cells (Figure 8d;24). The Bax/Bak (−/−) cell line treated with Tat-VP (C3) for 24 h is sensitive to the peptide and has the same percentage of all death as the control cell line on the criteria of plasma membrane permeabilization (7-AAD+) and ΔΨm loss (JC-1 low). This indicates that Tat-VP induces a Bax/Bak independent cell death.
Evaluation of lysosomal membrane permeabilization (LMP) and lysosomal catabolic proteases' role during TEAM-VP-induced apoptosis. (a) Absence of LMP induction in TEAM-VP-treated cells. HUVEC cells were treated with 10 μM TEAM-VP (24 h) or exposed for 1 min to UV and treated for 16 h with 10 μg/ml norfloxacin (NFX) (positive control for LMP). Fixed cells were labeled with anti-Cathepsin B (revealed Alexa fluor® 594, red fluorescence) and anti-Lamp2-FITC (lysosomes, green fluorescence) antibodies before observation by fluorescence microscopy. Fluorescent micrographs are representative for >95% of the cells. Scale bars, 12.5 μm. Note that in control untreated cells and in TEAM-VP-treated cells cathepsin B colocalizes with Lamp2 as demonstrated by the blend of the red and green fluorescence (yellow). In contrast, in the norfloxacin-treated cells cathepsin B becomes diffuse in the cytosol and does not codistribute with Lamp2 (no yellow fluorescence). Similar results were obtained using 20 μM TEAM-VP. (b) Effects of lysosomal proteases inhibition on TEAM-VP-induced ΔΨm dissipation and cell death. Cells were treated or not with Chloroquine plus NH4Cl (Clq; 100 μM and 20 mM, respectively) or Z-FA-fmk (Z-FA; 50 μM) for 1 h before addition of 10 μM TEAM-VP (24 h) and exposed to JC-1 dye and 7-AAD. Percentages of ΔΨm low cells and loss of viability are presented (mean±S.D., n=3)
Proapoptotic effects of TEAM-VP. (a) Multiparametric analysis, structural requirements and caspase-independence of TEAM-VP-mediated killing. HUVECs optionally cultured with 50 μM of Q-VD-OPH (red ‘+’) were treated with 15 μM of TEAM-VP, CycRGD or VP, followed by staining with JC-1 dye (after 16 h), Annexin-V-FITC (for determination of surface phosphatidylserine exposure, after 24 h of culture) or fixed with ethanol and stained with PI to determine the frequency of hypoploid cells (after 48 h). Flow cytometry data are pooled from three independent experiments (means±S.D.). (b) Induction of a caspase-3-like activity by TEAM-VP. Caspase-3 activity was measured by fluorimetry (Ac-DEVD-AMC cleavage; excitation: 360 nm; emission: 465 nm) in cytosolic fractions of HUVEC cells treated or not with 20 μM TEAM-VP for 24 h, in the presence (red ‘+’) or not of 20 μM Z-DEVD-fmk. The histogram shows fluorescence intensity in arbitrary units (a. u.; mean±S.D., n=3). (c) TEAM-VP-induced nuclear apoptosis. Upper panels show representative fluorescent micrographs for control untreated (Co.), stage I (peripheral chromatin condensation) and stage II (apoptotic bodies) nuclei of HUVEC cells stained with Hoechst 33342. Scale bars, 7.5 μm. The histograms show the frequency of stage I and stage II nuclei in HUVEC cells treated with TEAM-VP (10–30 μM) for 24 h (left panel) or with 20 μM TEAM-VP for 8, 16, 24 and 48 h in the presence (red ‘+’) or absence of 50 μM of Z-VAD-fmk (right panel). Lower and upper standard deviations are represented for stage I and stage II nuclei, respectively (mean±S.D., n=3). Note that (caspase-independent) stage I is the dominant morphotype of TEAM-VP-induced nuclear apoptosis. (d) Importance of Bcl-2 family proteins in Vpr-induced cell death. HCT-116 Bax +/− (Co.) and Bax/Bak −/− were treated or not with indicated concentrations of Tat-VP or 500 μM Sulindac sulfide (positive control;24) for 24 h and exposed to 7-AAD and JC-1 dye. Percentages of viability loss (left panel) and ΔΨm low cells (right panel) are presented (mean±S.D., n=3)
Discussion
The past 15 years have witnessed an explosion in the basic knowledge of mechanisms regulating apoptosis and the mediators that either trigger or inhibit cell death. Consequently, great interest has emerged in devising therapeutic strategies for modulating the key molecules of life-and-death decisions.25 The central role of mitochondrial membrane permeabilization (MMP) in mediating necrotic and (intrinsic-) apoptotic processes has led to the idea that mitochondria are reservoirs of potential targets for various therapeutic area including cancer therapy. Some antitumoral compounds with direct MMP-inducing effects have been validated in animal models. This is the case for delocalized lipophilic cations such as F16 or MKT-77.26
Two rational strategies can be distinguished in the design of anti-tumoral compounds acting directly on mitochondria. One is to identify a target that is present only in tumor mitochondria and then to screen (low molecular weight) drugs acting on this specific target. Although various PTP components or other mitochondria-associated proteins are selectively upregulated in tumor cells, druggable MMP-inducing compounds being truly selective for tumor mitochondria have still not been identified. A second strategy is to select natural or xenobiotic compounds/chemical structures having a potent MMP-inducing effect on any type of mitochondria, and then to deliver selectively such molecules into cancer cells or alternatively into tumor-related neovasculature. As a proof of concept for such targeting strategy, Ellerby et al.,6 have shown that when used in the systemic treatment of mice a chimeric peptide consisting of a tumor blood vessel ‘homing’ motif, linked to the (KLAKLAK)2 peptide that disrupts mitochondrial membranes, was selectively toxic to angiogenic endothelial cells and showed anticancer activity.
The present study describes in vitro and in cellula properties of a new chimeric peptide TEAM-VP which is composed of a cyclic RGD peptide motif and a small sequence derived from HIV-1 Vpr (termed VP). Using such a peptide we showed the following properties: (1) Both VP and TEAM-VP interact with ANT and/or VDAC to induce mitochondrial swelling, ΔΨm loss, cytochrome c release and reduction of oxygen consumption. (2) TEAM-VP efficiently kills αVβ3 expressing cells (i.e. HUVECs, HMVECd, A375M), but has no effect on αVβ3 negative cells including peripheral blood mononuclear cells. (3) TEAM-VP uptake in endothelial cells results from an active, energy-dependent process, and is followed by a transient lysosomal localization. (4) Then, TEAM-VP appears to leave lysosomes to reach the mitochondrial compartment, but does not trigger lysosomal membrane permeabilization (LMP). (5) TEAM-VP triggers mitochondrial hallmarks of apoptosis, and fragmentation of the mitochondrial network. (6) A stabilized version of TEAM-VP, TEAM-DVP (in which the VP part is composed of D-amino-acids) presents a stronger mitochondriotoxicity (SD50=70 nM) and cytotoxicity (LD50=6.8±1.5 μM) than TEAM-VP (the initial L-version).
The early internalization route of TEAM-VP in HUVEC cells is in the line with published reports on cycloRGDfK peptides,27, 28 thus indicating that a mitochondriotoxic part can be added without to put a damper on RGD binding and internalization through αVβ3 receptors. Distribution in lysosomes has already been observed for other RGD-peptides like cycloRGDfK,27, 28 but in our study confocal microscopy clearly shows that TEAM-VP reaches mitochondria after its release from lysosomes without degradation. This is the first detailed study showing microscopic evidence that RGD-containing peptide reaches its targeted intracellular compartment. It is unclear how TEAM-VP escapes from lysosomes. One hypothetic explanation comes from the observation at 24-h treatment of a massive fusion of lysosomes or late endosomes during which the vesicular membranes might be fragilized, thus allowing the peptide to escape. Another possibility would be related to membrane-destabilizing capabilities of the positively charged alpha-helical amphipathic VP-peptide structure.29, 30 Whatever is the case, there is no massive lysosomal membrane permeabilization in TEAM-VP-treated cells and cell death still occurs when lysosomal proteases are inhibited. This indicates that the TEAM-VP-induced apoptotic process is not characterized by an escape of active lysosomal proteases in the cytoplasm. In addition, TEAM-VP-induced cell death is not inhibited even partially by autophagy inhibitors like 3-methyladenine and bafilomycin A1 (data not shown) thus arguing against the possibility of an autophagy-related TEAM-VP-induced cell death.22, 31
Although Tat-VP does not require Bax/Bak to kill HCT-116 colon carcinoma cells, primary endothelial cell death triggered by TEAM-VP is characterized by classical apoptotic events such as mitochondrial changes (ΔΨm loss, cytochrome c release, fission), caspase-3 activation, phosphatidylserine exposure and nuclear apoptosis. There is evidence that some RGD-derived peptides might trigger apoptosis by direct caspase-3, -8 or -9 activation in an anchorage-independent manner.32, 33 Caspases-3 and -8 contain a potential RGD-binding motif allowing interaction with RGD peptides and subsequent autoprocessing and activation of these enzymes.32 Although it is not excluded that such an interaction exists with TEAM-VP, low or absent apoptosis hallmarks in HUVECs treated with the cyclic RGD domain alone or a VP-mutated version of TEAM-VP (see the control peptide termed C2) strongly suggest that the RGD sequence is not apoptogenic in the context of TEAM-VP.
When added to isolated mitochondria, TEAM-VP and TEAM-DVP are able to bypass the protective effects of cyclosporin A, and of the antiapoptotic Bcl-2 and Bcl-xl proteins. These results contrast with those obtained with Vpr1-96, Vpr52-96 and the shorter sequence Vpr71-82 that induce MMP in a CsA- and Bcl-2-dependent manner.10, 11 However, similar to native Vpr peptides,10, 11 TEAM-VP interacts with both ANT and VDAC (Figure 5f,g). Vpr peptides also form alpha-helical structures12, 30 and were shown to interact with planar lipid bilayers to form cation-selective channels.34 During initial screening of Vpr-derived peptides, we selected VP because it has the strongest efficacy to induce swelling and ΔΨm on isolated mitochondria. It is highly probable that minor modifications in the Vpr-sequence will influence the relative contributions of VDAC, ANT and mitochondrial lipids during mitochondrial membrane permeabilization induced by these peptides. Recently, Sabbah et al.12 using cross-linking experiment and surface plasmon resonance confirmed the Vpr/ANT interaction and proposed a tridimensionnal model for the Vpr–ANT complex. Interestingly docking program and dynamic simulations have led these authors to suggest that both Cys76 and Arg80 within Vpr are important for the interaction with ANT. This fits with our data since within TEAM-VP the cys76 is replaced by a serine, TEAM-VP interacts with ANT (Figure 5f,g) and both VP and TEAM-VP-induced swelling are insensitive to CsA (Figures 1a, 5a,b). When we used the mVP and TEAM-mVP (C2) control peptides in which C76 and R80 are replaced by Ser and Ala, respectively, we found that TEAM-mVP does not interact with ANT in HUVECs and that mVP and TEAM-mVP do not induce an MMP effect (Figures 1a,b, 5b).
These TEAM-VP peptide properties may be an important advantage from a therapeutic point of view since Bcl-2 overexpression is frequently a causal factor in tumor resistance to chemotherapy, and since Bcl-2 is also upregulated in angiogenic endothelial cells35, 36 and in αvβ3-expressing invasive melanoma (reviewed by Seftor et al37). It should be noted that when used in vitro to kill HUVECs, the TEAM-DVP LD50 appears 10 fold superior to the LD50 of the CNGRCGG-D[KLAKLAK]2 anticancer peptide described by Ellerby et al.6 As they have demonstrated efficacy in vivo in murine models of tumor growth, one can conclude that TEAM-DVP is an interesting candidate for further in vivo investigations.
Together, our data with TEAM-VP constitute the first example of viral-derived synthetic peptides capable of selectively killing αVβ3-expressing cells through a VDAC/ANT-related mitochondrial targeting. Hence, it will be of great interest to evaluate the in vivo efficacy of TEAM-VP (alone or combined with known anticancer agents) to control tumor growth. A number of viruses from various families encode proapoptotic sequences that directly interact with mitochondria.7, 8 It will be an exciting challenge to systematically explore viral genomes as a source of peptide-based structures to design new anticancer compounds acting directly on mitochondria.
Materials and Methods
Cells and apoptosis modulation
Human umbilical vein endothelial cells (HUVEC) and dermal microvascular endothelial cells (HMVEC-d) were obtained from BioWhittaker (Cambrex). HUVEC and HMVEC-d cells were maintained in culture in EBM-2 supplemented with the EGM-2 or EGM-2 MV Singlequots, respectively (Clonetics, Cambrex) according to manufacturer recommendations. Cells were used between passages 3 and 8. A375M, HeLa, HT-29, MCF7, MDA-MB-231 and Jurkat cell lines were obtained from American Type Cell Collection (ATCC). CEM clone 13 derived from human lymphoid cell line (CEM) was provided by Dr B Krust (UPR 2228 CNRS, France). HCT-116 Bax (+/−) and Bax/Bak (−/−) cell lines generated by Professor B Vogelstein (Johns Hopkins University) and Professor G Chinnadurai (Saint Louis University School of Medicine) were cultured in Mc Coy's 5A medium supplemented with 10% FCS. Lymphoid cells (CEM and Jurkat) were cultured in RPMI 1640 Glutamax supplemented with 10% FCS and antibiotics. Adherent cell lines were cultured in DMEM Glutamax supplemented with 10% FCS and antibiotics. Fresh human primary peripheral blood mononuclear cells (PBMCs) from healthy donors purified with Lymphoprep (Pharmacia) were cultured in RPMI 1640 Glutamax medium with 10% FCS. Various peptides were added directly to the medium (12- to 24-well plates) of exponentially growing cells. Chloroquine (Sigma Aldrich; 100 μM), NH4Cl (Sigma Aldrich; 20 mM), N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone (Z-FA-fmk), N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (Z-VAD-fmk) and Quinoline-Val-Asp(OMe)-CH2-O-Ph (Q-VD-OPH) (ICN; 50 μM) were added 1 h before addition of peptides. The epipodophyllotoxin derivative, etoposide (VP16) and peptide were added simultaneously to the cell culture. Lysosomal membrane permeabilization (LMP) was induced by treating HUVEC cells with norfloxacin (NFX; Sigma Aldrich; 10 μg/ml) for 16 h after a 1 min-exposure to UV radiation, 1 h after norfloxacin addition.22
Peptides and reagents
Vpr52–96, Vpr67–82, Vpr67–82[C76S] (termed VP), hBaxBH3(57–72[C62S]), hBidBH3(84–99) and Mastoparan (1–14), TEAM-VP and all variants or fragments described in Figure 2a were synthesized by Neosystem (Strasbourg, France) or alternatively Epytop (Nimes, France), using classical automated solid-phase synthesis and purification by reverse-phase HPLC. The synthesis of VP and TEAM-VP derivatives in D-configuration was carried out with the same strategy. All peptides were synthesized with carboxy-terminal amidation and with a free amino-terminal extremity or alternatively NH2-terminal biotinylation. RGD-containing peptides were or not subjected to cyclization via cystein oxidation. In some cases NH2-terminal FITC peptide conjugates were constructed. All peptides were analyzed by electrospray mass spectrometry and found to be >85% pure. Stock solution (sterile H2O, 1 mM) were prepared from lyophilized peptides, aliquoted and stored frozen at −80°C. MMP inhibitors used are: 4,4′-diisothiocyanastilbene-2,2′-difulfonic acid disodium salt (DIDS; Sigma Aldrich; 2–16 μM), cyclosporin A (CsA; BIOMOL Research Lab Inc.), recombinant Bcl-xl (Oncogene™ Research products; 1 μM) and recombinant Bcl-2 (Oncogene™ Research products; 1 μM).
Preparation of mitochondria and assessment of mitochondrial parameters
Liver mitochondria were isolated from 3–4 week old Balb-c mice (IFFA CREDO) as described previously.38 Mitochondria were also isolated from HeLa cells (3 × 106 cells) using a protocol adapted from reference.39 Briefly, cells were harvested with PBS containing 1 mM EDTA, centrifuged at 750 g for 10 min, washed and resuspended in isotonic MB buffer (210 mM mannitol, 70 mM sucrose, 1 mM EDTA and 10 mM HEPES, pH 7.5) supplemented with protease inhibitors. Cells were broken by 10 passages through a 27G × 3/4″ 0.4 × 20 mm needle fitted on a 2.5 ml-syringe. The suspension was centrifuged at 1700 × g at 4°C for 2 min. Cell break was observed by microscopy. Supernatants were pooled and centrifuged at 10 000 × g at 4°C for 10 min and mitochondrial pellet resuspended in MB buffer at 2 mg/ml of proteins. For detection of large amplitude swelling, liver mitochondria (100 μg of protein/ml) were incubated for 15–30 min with peptides or MMP-inducing drugs in 1 ml buffer A (200 mM sucrose, 1 mM H3PO4, 5 mM succinate, 10 mM MOPS, 2 μM rotenone and 10 μM EGTA, pH 7.4). Swelling was determined by measuring absorbance at 545 nm with an Ultrospec 3300 pro spectrofluorometer (Amersham Pharmacia Biotech).38 The percentage of specific swelling was calculated as follows: (Ainit−Areagent) 100/(Ainit−ACa2+) where ACa2+, Areagent and Ainit correspond to the absorbance value obtained for CaCl2-treated, reagent-treated and untreated mitochondria, respectively. Mitochondrial transmembrane potential (ΔΨm) was assessed by incorporation of 5,5′,6,6′,-tetrachloro 1,1,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1; excitation 488 nm; emission 525–535 nm (low ΔΨm) and 560–605 nm (high ΔΨm); Molecular Probes; 1 μM) and flow cytometry acquisition with a FACScalibur (Becton Dickinson) as described previously.38 For the determination of cytochrome c release, mitochondria (30 μg proteins) isolated from liver were incubated with 5 μg/ml Alamethicin (positive control) or 1 μM of peptide in the presence or not of DIDS (5 μM) in buffer A for 30 min at RT. After centrifugation at 6800 × g for 10 min, the mitochondrial pellet was frozen for subsequent analysis. Alternatively, mitochondria (30 μg proteins) isolated from HeLa cells were incubated in the presence of t-Bid (gift from Professor JC Martinou) or peptide for 20 min at 37°C in buffer B (125 mM KCl, 4 mM MgCl2, 5 mM NaHPO4, 5 mM succinate, 0.5 mM EGTA, 15 mM Hepes, pH 7.4 and 5 μM rotenone). After a 7 min-centrifugation at 10 000 × g, proteins contained in supernatant and in resuspended mitochondrial pellet were run on SDS–PAGE (12%) and transferred to Hybond-P (Amersham Biosciences) in a Mini Protean II system (Biorad) for 1 h at 100 V. Subsequently the membrane was blocked for 1 h with 5% low fat milk in TBS-0.1% tween-20 (TBST) and incubated overnight with anti-cytochrome c monoclonal antibody (BD Bioscience Pharmingen; 1/500). After 1 h washes with TBST, the membrane was treated with horseradish peroxidase-coupled anti-mouse IgG (Amersham; 1/2000) for ECL detection (Amersham).
Affinity purification of PTP components
For trapping on isolated mitochondria, mouse liver mitochondria (250 μg of protein/100 μl, in buffer A) were incubated for 20 min at RT with 5 μM of biotinylated peptides. Mitochondria were washed once in buffer A and the pellet was lysed with 150 μl of buffer C (20 mM Tris-HCl, pH 7.6, 400 mM NaCl, 50 mM KCl, 1 mM EDTA, 0.2 mM PMSF, aprotinin (100 U/ml), 1% triton X-100 and 20% glycerol). For trapping on HUVECs, the cells were treated for 24 h with 15 μM of biotinylated peptides. Cells were harvested and lysed with 150 μl of buffer C. The liver mitochondrial extracts and HUVEC cellular extracts were diluted with 300 μl PBS plus 1 mM EDTA before addition of 50 μl streptavidin-agarose (Pierce). After 2 h incubation at 4°C in a roller drum, the beads were washed (5 ×) batchwise with PBS plus 1 mM EDTA and resuspended in 50 μl of 2 × Laemmli sample buffer. Proteins were analyzed by Western blot using (1) for liver mitochondria: mouse monoclonal IgG2b anti-Porin 31HL (VDAC; clone Ab-2; Calbiochem; 1/2000), goat polyclonal IgG anti-ANT (Q18; Santa Cruz; 1/500) or mouse monoclonal IgG anti-Hsp60 (clone LK1; Sigma; 1/1000) and (2) for HUVEC: mouse monoclonal IgG anti-Hsp60 (clone LK1; Sigma; 1/1000) or rabbit polyclonal IgG anti-hANT. We obtained this anti-hANT polyclonal antibody after immunization of rabbits with the 21-mer peptide GVYDTAKGMLPDPKNVHIFVS corresponding to the human isoform 1 of ANT, region 193–213; 1/500).
Assessment of apoptosis parameters
For ΔΨm dissipation determination, the JC-1 dye (1 μM; Molecular Probes) was used; for plasma membrane permeabilization assessment, 7-Aminoactinomycin D (10 μg/ml; 7-AAD; Sigma Aldrich) was employed; for phosphatidylserine exposure determination, human recombinant Annexin-V-FITC (1/100; Immunotech) was used. The hypoploidy frequency was determined by propidium iodide (PI, 50 μg/ml) staining of ethanol-permeabilized cells treated with RNase (Sigma Aldrich, 500 ng/ml) supplemented with 5 mM glucose for 1 h at RT. For each sample, around 105 cells were analyzed by flow cytometry with a FACSCalibur cytometer (Becton Dickinson) and the CellQuest pro software (Becton Dickinson). For in situ detection of cytochrome c release, fixed and permeabilized cells were incubated with mouse monoclonal IgG2b anti-Porin 31HL (VDAC; clone Ab-2; Calbiochem; 1/200) and mouse monoclonal IgG1 anti-cytochrome c (clone 6H2.B4; BD Pharmingen; 1/200) for 1 h at RT. After washes with PBS, goat anti-mouse IgG2b Alexa Fluor® 594 (Molecular Probes; 1/200) and goat anti-mouse IgG1-FITC (Sigma; 1/200) were added for 1 h at RT. Cells were mounted in Vectashield (Vector Laboratories Inc.) and observed on an inverted Leica DM IRB microscope. Alternatively, mitochondria were labeled with 60 nM MitoTracker® green (Molecular Probes) and fission detected by fluorescence microscopy. For caspase-3 fluorimetric assay, cytosolic extracts were prepared as described.40 Cytosolic proteins (4 μg) were preincubated for 15 min at 37°C with or not N-benzyloxycarbonyl-Asp-Glu(OMe)-His-Asp(OMe)-fluoromethylketone (Z-DEVD-fmk; ICN; 20 μM) before addition of caspase-3 substrate Ac-DEVD-AMC (BIOMOL Research Lab Inc.; 10 μM) for 2 h at 37°C in a 200 μl-final volume. Fluorescence was measured (excitation 360 nm; emission 465 nm) using a Genios spectrofluorometer (Tecan).
Phenotyping and binding to integrins
HUVEC cells were trypsinized, washed with PBS and fixed in 1% paraformaldehyde in PBS with 1% BSA and 0.1% NaN3 for 30 min at 4°C. After washes with PBS cells were incubated for 30 min at RT with PE-conjugated mouse monoclonal anti-αVβ3 (clone 23C6; BD Pharmingen; 1/25) or PE-conjugated mouse monoclonal anti-αVβ5 (clone P1F6; Chemicon; 1/50) or mouse IgG1 R-PE control (Cymbus Biotechnology LTD) in PBS with 1% BSA and 0.1% NaN3. Cells were washed and analyzed for integrin expression levels by flow cytometry. For binding assays, cells were incubated for 45 min at RT or 4°C with 0.5–2 μM FITC-labeled peptide. For competition assays, cells were preincubated for 30 min at RT with unlabeled competitive peptides in excess before addition of FITC-labeled peptide for a 45 min-incubation. In each case, cells were rinsed with PBS, trypsinized, washed twice with PBS and analyzed by flow cytometry.
Peptide routing
Peptide internalization by endocytosis was studied by treating cells for 15 min-5 h with 2.5 μM peptide and 1 mg/ml rhodamine B isothiocyanate-dextran 40S beads (Sigma Aldrich) after or not a 30 min-pretreatment with 5 mM NaN3 and 50 mM 2-deoxyglucose inducing ATP depletion. Cells were labeled with Hoechst 33342 (Sigma Aldrich; 2 μg/ml) before observation on an inverted Leica DM IRB fluorescence microscope. For immunofluorescence analysis, peptide-treated cells were fixed in 4% paraformaldehyde in PBS for 30 min at 4°C and permeabilized for 5 min with 0.1% triton X-100 in PBS. Cells were incubated with either mouse monoclonal IgG2b anti-Porin 31HL (VDAC; clone Ab-2; Calbiochem; 1/200), mouse monoclonal IgG1 anti-Lamp2-FITC CD107b (clone H4B4; BD Pharmingen; 1/5), mouse monoclonal IgG1 anti-Golgin-97 (Molecular Probes; 1/100) or mouse monoclonal IgG2aκ anti-Cathepsin B (clone CA10; Oncogene Research Products; 1/250) in PBS with 0.5% BSA and 2% FCS for 1 h at RT. After washes with PBS, anti-mouse IgG Alexa Fluor® 546 (Molecular Probes; 1/200; for anti-Porin), anti-mouse IgG Fluorescein (Molecular Probes; 1/100; for anti-Golgin) or anti-mouse IgG Alexa Fluor® 594 (Molecular Probes; 1/200; for anti-Cathepsin) was added and/or Streptavidin-Texas Red (Molecular Probes; 4 μg/ml) or Streptavidin-FITC (Sigma Aldrich; 4 μg/ml) in PBS with 0.5% BSA and 2% FCS for 1 h incubation. Cells were mounted in Vectashield (Vector Laboratories Inc.) and observed on either an inverted Leica DM IRB fluorescence microscope (HCX PL Fluotar oil immersion objective 100 ×) or a Zeiss confocal microscope LSM 510 (Plan Apochromat oil immersion objective 63 × NA 1.4). Deconvolution analysis was carried out using Huygens Professional 2.6a64 software on SGI 3400. Three-dimensional reconstruction was realized using Imaris version 4.05 (Bitplane) software.
Electron microscopy
Isolated mitochondria were fixed in 3.2% glutaraldehyde in 0.1 M Soerensen buffer (0.1 M phosphate, pH 7.2; Prolabo) for 16 h at 4°C, then sample was prepared as described.38 HUVEC cells were fixed with 2% glutaraldehyde in 0.1 M Nacacodylate buffer, pH 7.2 for 3 h at 4°C. After washes in the same buffer, specimens were then postfixed with 1% osmium tetroxide containing 1.5% potassium cyanoferrate, dehydrated in gradual ethanol (30–100%) and embedded in Epon. Thin sections (70 nm) were collected onto 200 mesh cupper grids, counterstained with uranyl acetate and lead citrate before examination with a Philips CM12 transmission electron microscope at 80 kV.
Polarographic study
Purified mitochondria were incubated in a magnetically stirred 1.5 ml cell with a Clark type oxygen electrode (Hansatech) thermostated at 37°C, in 500 μl of a medium consisting of 0.3 M mannitol, 10 mM phosphate buffer (pH 7.3), 10 mM KCl, 5 mM MgCl2 and 1 mg/ml BSA as previously described.41 Respiratory chain complexes activities were measured 4 min after TEAM-VP addition using standard procedure.41
Abbreviations
- ANT:
-
adenine nucleotide translocator
- Clq:
-
chloroquine
- CsA:
-
cyclosporin A
- ΔΨm:
-
mitochondrial transmembrane potential
- DIDS, 4,4′-diisothiocyanastilbene-2:
-
2′-difulfonic acid disodium salt
- ED50:
-
effective dose giving half of the maximal effect
- FITC:
-
fluorescein isothiocyanate
- HIV:
-
human immunodeficiency virus
- HUVEC:
-
human umbilical vein endothelial cells
- JC-1, 5,5′,6,6′-tetrachloro-1,1′, 3:
-
3′-tetraethylbenzimidazolylcarbocyanine iodide
- LMP:
-
lysosomal membrane permeabilization
- mClCCP:
-
m-chlorocarbonylcyanide phenylhydrazone
- MMP:
-
mitochondrial membrane permeabilization
- NFX:
-
norfloxacin
- PE:
-
phycoerithrine
- PI:
-
propidium iodide
- PTP:
-
permeability transition pore
- Q-VD-OPH:
-
Quinoline-Val-Asp(OMe)-CH2-O-Ph
- VDAC:
-
voltage-dependent anion channel
- Vpr:
-
viral protein R
- Z-DEVD-fmk:
-
N-benzyloxycarbonyl-Asp-Glu(OMe)-His-Asp(OMe)-fluoromethylketone
- Z-FA-fmk:
-
N-benzyloxycarbonyl-Phe-Ala-fluoromethylketone
- Z-VAD-fmk:
-
N-benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone
References
Carmeliet P, Jain RK . Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–257.
Ruoslahti E . RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 1996; 12: 697–715.
Brooks PC, Clark RA, Cheresh DA . Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994; 264: 569–571.
Hart SL, Knight AM, Harbottle RP, Mistry A, Hunger HD, Cutler DF et al. Cell binding and internalization by filamentous phage displaying a cyclic Arg-Gly-Asp-containing peptide. J Biol Chem 1994; 269: 12468–12474.
Arap W, Pasqualini R, Ruoslahti E . Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998; 279: 377–380.
Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med 1999; 5: 1032–1038.
D'Agostino DM, Bernardi P, Chieco-Bianchi L, Ciminale V . Mitochondria as functional targets of proteins coded by human tumor viruses. Adv Cancer Res 2005; 94: 87–142.
Boya P, Pauleau AL, Poncet D, Gonzalez-Polo RA, Zamzami N, Kroemer G . Viral proteins targeting mitochondria: controlling cell death. Biochim Biophys Acta 2004; 1659: 178–189.
Muthumani K, Choo AY, Premkumar A, Hwang DS, Thieu KP, Desai BM et al. Human immunodeficiency virus type 1 (HIV-1) Vpr-regulated cell death: insights into mechanism. Cell Death Differ 2005; 12 (Suppl 1): 962–970.
Jacotot E, Ravagnan L, Loeffler M, Ferri KF, Vieira HL, Zamzami N et al. The HIV-1 viral protein R induces apoptosis via a direct effect on the mitochondrial permeability transition pore. J Exp Med 2000; 191: 33–46.
Jacotot E, Ferri KF, El Hamel C, Brenner C, Druillennec S, Hoebeke J et al. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein rR and Bcl-2. J Exp Med 2001; 193: 509–519.
Sabbah EN, Druillennec S, Morellet N, Bouaziz S, Kroemer G, Roques BP . Interaction between the HIV-1 protein Vpr and the adenine nucleotide translocator. Chem Biol Drug Des 2006; 67: 145–154.
Jacotot E, Deniaud A, Borgne-Sanchez A, Touat Z, Briand JP, Le Bras M et al. Therapeutic peptides: targeting the mitochondrion to modulate apoptosis. Biochim Biophys Acta 2006, in press.
Szabo I, De Pinto V, Zoratti M . The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett 1993; 330: 206–210.
Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ . Cyclophilin D as a drug target. Curr Med Chem 2003; 10: 1485–1506.
Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004; 427: 461–465.
Pfeiffer DR, Gudz TI, Novgorodov SA, Erdahl WL . The peptide mastoparan is a potent facilitator of the mitochondrial permeability transition. J Biol Chem 1995; 27: 4923–4932.
Andrieu M, Loing E, Desoutter JF, Connan F, Choppin J, Gras-Masse H et al. Endocytosis of an HIV-derived lipopeptide into human dendritic cells followed by class I-restricted CD8(+) T lymphocyte activation. Eur J Immunol 2000; 30: 3256–3265.
Sandvig K, Olsnes S . Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem 1982; 257: 7504–7513.
Wadia JS, Stan RV, Dowdy SF . Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med 2004; 10: 310–315.
Martinou JC, Youle RJ . Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ 2006; 13: 1291–1295.
Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D et al. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 2003; 197: 1323–1334.
Susin SA, Daugas E, Ravagnan L, Samejima K, Zamzami N, Loeffler M et al. Two distinct pathways leading to nuclear apoptosis. J Exp Med 2000; 192: 571–580.
Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B . Role of BAX in the apoptotic response to anticancer agents. Science 2000; 290: 989–992.
Fischer U, Schulze-Osthoff K . New approaches and therapeutics targeting apoptosis in disease. Pharmacol Rev 2005; 57: 187–215.
Bouchier-Hayes L, Lartigue L, Newmeyer DD . Mitochondria: pharmacological manipulation of cell death. J Clin Invest 2005; 115: 2640–2647.
Castel S, Pagan R, Mitjans F, Piulats J, Goodman S, Jonczyk A et al. RGD peptides and monoclonal antibodies, antagonists of alpha(v)-integrin, enter the cells by independent endocytic pathways. Lab Invest 2001; 81: 1615–1626.
Schraa AJ, Kok RJ, Berendsen AD, Moorlag HE, Bos EJ, Meijer DK et al. Endothelial cells internalize and degrade RGD-modified proteins developed for tumor vasculature targeting. J Control Release 2002; 83: 241–251.
Coeytaux E, Coulaud D, Le Cam E, Danos O, Kichler A . The cationic amphipathic alpha-helix of HIV-1 viral protein R (Vpr) binds to nucleic acids, permeabilizes membranes, and efficiently transfects cells. J Biol Chem 2003; 278: 18110–18116.
Morellet N, Bouaziz S, Petitjean P, Roques BP . NMR structure of the HIV-1 regulatory protein VPR. J Mol Biol 2003; 327: 215–227.
Gonzalez-Polo RA, Boya P, Pauleau AL, Jalil A, Larochette N, Souquere S et al. The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci 2005; 118: 3091–3102.
Buckley CD, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D et al. RGD peptides induce apoptosis by direct caspase-3 activation. Nature 1999; 397: 534–539.
Aguzzi MS, Giampietri C, De Marchis F, Padula F, Gaeta R, Ragone G et al. RGDS peptide induces caspase 8 and caspase 9 activation in human endothelial cells. Blood 2004; 103: 4180–4187.
Piller SC, Ewart GD, Premkumar A, Cox GB, Gage PW . Vpr protein of human immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. Proc Natl Acad Sci USA 1996; 93: 111–115.
Gerber HP, Dixit V, Ferrara N . Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998; 273: 13313–13316.
Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM et al. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res 2001; 61: 2183–2188.
Seftor RE, Seftor EA, Hendrix MJ . Molecular role(s) for integrins in human melanoma invasion. Cancer Metastasis Rev 1999; 18: 359–375.
Lecoeur H, Langonne A, Baux L, Rebouillat D, Rustin P, Prevost MC et al. Real-time flow cytometry analysis of permeability transition in isolated mitochondria. Exp Cell Res 2004; 294: 106–117.
Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 1999; 144: 891–901.
Enari M, Hase A, Nagata S . Apoptosis by a cytosolic extract from Fas-activated cells. EMBO J 1995; 14: 5201–5208.
Rustin P, Chretien D, Bourgeron T, Gerard B, Rotig A, Saudubray JM et al. Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 1994; 228: 35–51.
Acknowledgements
We thank Dr Sylviane Muller for her critical reading of the manuscript and Dr Naoufal Zamzami for helpful suggestions. We are grateful to Dr Peter Daniel for kindly providing Bax (+/−) and Bax/Bak (−/−) colon cancer cells generated by Professor B Vogelstein (Johns Hopkins University) and Professor G Chinnadurai (Saint Louis University School of Medicine). This work was supported by grants from the French Ministry of Research (GenHomme Programs 2001 and 2003) to CB (No 01H0480 and No 03L297) and EJ (No 01H0476 and No 03L292), by Agence Nationale pour la Valorisation de la Recherche (ANVAR) to EJ (No R0209333Q and No A0404096Q), by the Association Française contre les Myopathies (AFM) to PR, and by Centre National de la Recherche Scientifique (CNRS) and Institut National de la Santé et de la Recherche Médical to PR. DR was supported by ANVAR (No K0109377Q), AL by Centre Régional d'Innovation et de Transfert de Technologie (CRITT) d'Ile de France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by JC Martinou
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Borgne-Sanchez, A., Dupont, S., Langonné, A. et al. Targeted Vpr-derived peptides reach mitochondria to induce apoptosis of αVβ3-expressing endothelial cells. Cell Death Differ 14, 422–435 (2007). https://doi.org/10.1038/sj.cdd.4402018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4402018
Keywords
This article is cited by
-
New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury
Angiogenesis (2020)
-
Magnetic nanotherapeutics for dysregulated synaptic plasticity during neuroAIDS and drug abuse
Molecular Brain (2016)
-
RETRACTED ARTICLE: RGD Peptide-Pegylated PLLA Nanoparticles Containing Epirubicin Hydrochloride Exhibit Receptor-Dependent Tumor Trafficking In Vitro and In Vivo
Pharmaceutical Research (2015)
-
Intracellular targets of RGDS peptide in melanoma cells
Molecular Cancer (2010)
-
A flavivirus protein M-derived peptide directly permeabilizes mitochondrial membranes, triggers cell death and reduces human tumor growth in nude mice
Apoptosis (2009)