Abstract
Enzymatic modification of low-density lipoprotein (LDL) as it probably occurs in the arterial intima drastically increases its cytotoxicity, which could be relevant for the progression of atherosclerotic lesions. LDL was treated with a protease and cholesterylesterase to generate a derivative similar to lesional LDL, with a high content of free cholesterol and fatty acids. Exposure of endothelial cells to the enzymatically modified lipoprotein (E-LDL), but not to native or oxidized LDL, resulted in programmed cell death. Apoptosis was triggered by apoptosis signal-regulating kinase 1 dependent phosphorylation of p38. Depletion and reconstitution experiments identified free fatty acids (FFA) as the triggers of this pathway. Levels of FFA in native LDL are low and the lipoprotein is therefore not cytotoxic; enzymatic cleavage of cholesterylesters liberates FFA that can rapidly trigger an apoptosis signaling cascade in neighboring cells. Blockade of this pathway can rescue cells from death.
Similar content being viewed by others
Introduction
A role for endothelial cell apoptosis in atherogenesis, plaque erosion and acute coronary syndromes is being discussed. 1, 2, 3 However, triggering events have not been unequivocally identified, and underlying molecular pathways remain largely unknown. Oxidized LDL (ox-LDL) and oxysterols can induce apoptosis of cells in vitro, but it is unclear whether levels of these substances reach significance in vivo. Free fatty acids (FFA) can induce apoptosis of cultured endothelial cells.4 However, conventional concepts of atherosclerosis do not envision large amounts of FFA to be present in atherosclerotic lesions. Exposure of endothelial cells to native LDL leads to phosphorylation of p38,5 a molecule that can be involved in apoptosis signaling pathways. However, LDL is not cytotoxic under physiological conditions, and therefore the relevance of p38 phosphorylation provoked by LDL has not been considered in the context of cell death.
A priori, it seems reasonable to assume that one or several constituents of LDL are responsible for triggering apoptosis of cells in and around intimal lipid deposits, so a key question concerns the nature of the modification that LDL undergoes in tissues. While oxidative events are generally believed to be most important, there are other possibilities that merit consideration. Lesion-born lipoprotein has long been known to have a high content of free cholesterol,6 to activate complement,7 and to induce macrophage foam cell formation.8, 9 Combined treatment of LDL with a protease and cholesterylesterase in vitro generates an LDL-derivative (E-LDL) with the same chemical, functional and micromorphological properties.10 LDL-modifying proteases11, 12, 13 as well as cholesterylesterase12 have been detected in atherosclerotic lesions, where they colocalize with enzymatically modified LDL, C-reactive protein (CRP) and complement.11, 14, 15 These findings indicate that nonoxidative, enzymatic remodeling of LDL occurs at the early stages of atherogenesis. We are submitting for discussion that this modification is not primarily harmful, but that it may be physiological and meaningful because the lipoprotein could thus be able to signal to the immune system and effect its own removal. Only when the capacity of the cholesterol removal system is overburdened are detrimental inflammatory events triggered that drive the development of advanced lesions and plaque instability.16 Apoptotic events likely become relevant at these later stages.
That extracellular lipid deposits in early atherosclerotic lesions have a high content of free cholesterol betrays that cholesterylesterase must be active at these sites, and this must be accompanied by the local generation of FFA. It followed that fatty acids generated within E-LDL might be directly capable of triggering apoptosis, and the present investigation was undertaken to examine this possibility. Affirmative results were obtained and a major pathway leading to cell death was identified. The results may also be relevant in other pathological scenarios where cells are confronted with supraphysiological amounts of FFA.
Results
E-LDL triggers apoptosis in endothelial cells
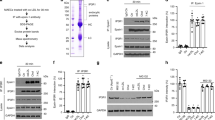
Caspases 3 and 7 belong to the subset of executioner caspases that are usually involved in programmed cell death. Caspase 3/7 activity was measured in lysates of endothelial cells treated with native or modified LDL. Native LDL and ox-LDL were without significant effect as compared to medium alone, but incubation with E-LDL resulted in more than 10-fold enhancement of caspase activity (Figure 1a). The stimulatory effect could be reduced through the removal of FFA. Removal of approximately 50% through incubation with 4% albumin (E-LDL(d4)) reduced caspase activity by approximately 40%, and removal of 90% FFA through incubation with 10% albumin (E-LDL(d10)) reduced caspase activity substantially further.
(a) Enzymatically modified LDL activates executioner caspases: dependence on FFA content. Endothelial cells were seeded into 96-well plates 1 day prior to treatment for 1 h with 100 μg/ml of the lipoprotein preparations indicated in the Figure. Caspase-Glo™ – 3/7 assays were performed after incubation for 3 h at 37°C. The data represent the means from three independent experiments, and indicate fold activation over untreated controls. (b) Annexin-V binds to endothelial cells after brief exposure to E-LDL. Endothelial cells were incubated with LDL (200 μg/ml) (left panel), or the same amount of E-LDL for 10 min and subsequently stained with Annexin-V-EGFP and DAPI; digital images were taken immediately after staining. (c) E-LDL induces DNA fragmentation. Endothelial cells were incubated with LDL, ox-LDL or E-LDL (75 μg/ml) or LA (free acid, 0.3 μM), and TUNEL assays were performed 16 h later. Representative photomicrographs are depicted. (d) E-LDL is cytotoxic. Endothelial cells were treated for 1 h with medium alone (control) or lipoproteins at 100 μg/ml as indicated in the Figure Intracellular ATP was measured after an additional incubation for 3 h at 37°C (n=6). (e) Dose dependent ATP-reduction by E-LDL. The experiment was principally performed as in (c) with various concentrations of E-LDL (n=3)
Exposure of phosphatidylserine on the outer leaflet of the plasma membrane as a second indicator of apoptosis was investigated using EGFP-labeled annexin V. As shown in Figure 1b, control cells that had been exposed to 200 μg/ml native LDL bound essentially no annexin V, whereas bright surface fluorescence was observed on cells after only 10 min incubation with E-LDL.
Caspase activation does not invariably indicate apoptotic cell death, and Annexin V- binding represents an early event in PCD. In order to provide firm evidence that E-LDL indeed causes apoptotic cell death in EC, we performed DNA-fragmentation assays using a fluorometric TUNEL-system. As shown in Figure 1c, cells treated with E-LDL, or linoleic acid (LA) yielded positive staining and nuclear fragmentation, while medium controls, native- or oxLDL remained negative.
Quantification of cellular ATP concluded this first experiment. There was no alteration in total ATP content of cells following incubation with 100 μg/ml of either native or ox-LDL for 1 h under serum-free conditions and subsequent 3 h incubation in medium containing 1% FCS. In contrast, cells that had been exposed to E-LDL exhibited reduction in cellular ATP of 40–50% (Figure 1d). The dose dependency of E-LDL induced ATP reduction is documented in Figure 1e.
FFA in LDL preparations rapidly induce p38-phosphorylation
p38 is a MAP-kinase that is involved in pathways leading to programmed cell death. One previous study showed that incubation of endothelial cells with native LDL resulted in p38 phosporylation.5 The underlying cause was not identified and, understandably, no link was made to the triggering of apoptosis because native LDL is not generally considered to be cytotoxic. At this juncture, we suspected that FFA might have represented the trigger, and that the extent of p38 phosphorylation might be enhanced in cells incubated with E-LDL.
As depicted in Figure 2a, incubation of endothelial cells with native LDL did lead to weak p38 phosphorylation, and unexpectedly, the results were no different following incubation with ox-LDL. In contrast, incubation with E-LDL resulted in a conspicuous enhancement of phosphorylation. Depletion of FFA from the lipoprotein preparations by incubation with 10% albumin markedly reduced the extent of p38 phosphorylation (Figure 2b).
(a) Rapid phosphorylation of p38 upon treatment of endothelial cells with E-LDL. Endothelial cells were exposed for 10 min to 100 μg/ml of native LDL, ox-LDL or enzymatically modified LDL. Medium alone (control), or sorbitol, served as negative and positive controls, respectively. Phosphorylated p38 (upper panel) and total p38 (lower panel) in whole-cell lysates were sequentially detected by Western blot on the same membrane. (b) Attenuation of E-LDL-mediated p38-phosphorylation by removal of FFA. Cells were incubated with 100 μg/ml LDL or E-LDL, or with lipoprotein preparations that had been depleted of FFA by incubation with 10% albumin, denoted LDL(d10) and E-LDL(d10). Western blot with anti-phospho-p38 (upper panel) and anti-p38 (lower panel) was performed as in Figure 2a
Application of LA, oleic acid (OA) or arachidonic acid alone also resulted in phosphorylation of the MAP-kinase (Figure 3a). As with E-LDL, phosphorylation occurred very rapidly, the band becoming strongly visible after only 10 min incubation and receding in intensity thereafter.
(a) Rapid, transient induction of p38 phosphorylation in endothelial cells by purified FFA. Endothelial cells were incubated with 100 μM linoleic acid (LA), oleic acid (OA), or arachidonic acid (AA), or with the carrier methyl-beta-cyclodextrin (Cy) for the indicated times, and cell lysates were analyzed for p38-phosphorylation (upper panel); lower panel shows p38 (b) Reconstitution experiments confirm that induction of p38-phosphorylation is mediated by FFA. Cells were treated with sorbitol (positive control) with 100 μg/ml E-LDL or LDL, or with lipoproteins depleted of FFA by incubation with 4% albumin (d4) or 10% albumin (d10), or with the latter preparations that had been reconstituted with linoleic acid (RE-LDL) (see Materials and Methods for details). Depletion of FFA from lipoproteins reduced p38 phosphorylation, and reconstitution with linoleic acid reconstituted p38 phosphorylating activity
Reconstitution experiments were then performed in which LA was added to FFA-depleted E-LDL (Figure 3b). The results supported the contention that the stimulatory effect of E-LDL was indeed invoked by fatty acids.
FFA-induced phosphorylation of p38 occurs via the apoptosis signaling kinase-1
p38 activation occurs via MAPKK3 or MAPKK6.17 Upstream of these are several MAPKKKs including apoptosis signal-regulating kinase 1 (ASK1), a kinase for which a role in the induction of apoptosis has been documented.18, 19 We decided to test whether blocking of ASK1 by siRNA mediated gene silencing might interfere with p38 phosphorylation induced by E-LDL. Since endogenous levels of ASK1 were too low to allow reliable detection in endothelial cells, COS-cells transfected with ASK1 were used to establish the efficacy of siRNA. While no single siRNA alone proved efficient in silencing ASK1, a cocktail composed of the two siRNA was found to be effective (Figure 4a). As shown in Figure 4b, treatment of control cells with LDL or E-LDL again led to phosphorylation of p38; this effect was almost completely abrogated by prior transfection of specific anti-ASK1 siRNA (SP). An unspecific control siRNA (UN) was without effect.
(a) Synthetic siRNA for ASK1 silence expression of transiently transfected FLAG-ASK1. Two different siRNA were synthesized that were designed to block ASK1 expression, and tested by cotransfection with FLAG-ASK1 and subsequent Western-blotting with anti-ASK1 antibodies. Numbers above the panels indicate the individual siRNA used for silencing. While no single siRNA was highly efficient, transfection with a mixture of both siRNA led to satisfactory silencing of gene expression. (b) ASK1-siRNA block phosphorylation of p38 in response to LDL and E-LDL. A Western blot with antibodies against p38 is shown. Endothelial cells were transfected with specific ASK1-siRNA (SP) or with an unspecific control-siRNA (UN) and then treated with lipoproteins. Lysates were analyzed by Western blotting for phosphorylated p38 (top panel) and total p38 (lower panel)
These results indicated that ASK1 was involved in E-LDL induced p38 phosphorylation, and it followed that ASK1 itself should be phosphorylated as a consequence of exposure of cells to the lipoproteins. This assumption was confirmed in Western blot analyses employing an antibody specific for phosphorylated ASK1. Cells transiently transfected with ASK1 cDNA were treated with lipoproteins and cell extracts were analyzed. In the experiment of Figure 5a, H2O2 was used as a positive control, and LDL or E-LDL were employed prior to or following FFA depletion (d10) with 10% albumin. The upper panel depicts phosphorylated ASK1, and the lower panel total ASK1. It is evident that ASK1 phosphorylation again occurred in dependence of the presence of FFA in LDL and E-LDL.
(a) FFA associated with E-LDL lead to phosphorylation of threonin 845 in ASK1. COS-7 cells were transiently transfected with ASK1 cDNA and treated with 100 μg/ml lipoprotein preparations for 10 min. Native LDL and E-LDL as well as respective lipoprotein preparations that have been depleted of FFA (d) by incubation with 10% albumin were employed. Whole-cell lysates were obtained and Western blots were performed using polyclonal antibodies against the phosphorylated ASK1 (upper panel) or against ASK1 (lower panel). (b) Purified FFA also induce ASK1 phosphorylation. The experiment was performed essentially as the one shown in Figure 5a, but 100 μM purified linoleic acid (LA), arachidonic acid (AA) or oleic acid (OA) were employed. The blot was developed with the antibody against phospho-ASK1
A further experiment was conducted with purified FFA. Figure 5b depicts a Western blot experiment in which LA, arachadonic acid (AA) or OA were applied at 100 μM. Again, the isolated fatty acids also were found to induce ASK1 phosphorylation.
Blockade of ASK1/p38 rescues cells
The above results indicated that FFA contained in E-LDL might trigger apoptosis via ASK1 and p38. First we employed the siRNA approach to put this assumption to test. As depicted in Figure 6a, E-LDL-induced caspase3/7 activation was completely suppressed by ASK1 siRNAs. In order to confirm that blockade of ASK1 also affects late steps of PCD, cleavage of PARP was assessed. As depicted in Figure 6b, the PARP p85 fragment was induced by treatment with LA or E-LDL, and this effect was significantly reduced by siRNAs targeting ASK1. The importance of p38 for the proapototic effect of E-LDL was demonstrated with the use of a specific inhibitor, SB203580. Cells were preincubated with the inhibitor at 20 μM for 2 h and then exposed to toxic concentrations of E-LDL. As shown in Figure 6c, caspase activity induced by E-LDL was almost halved by SB203580 pretreatment. Concomitantly, cytotoxicity as reflected by cellular ATP levels was almost totally abrogated.
Inhibition of ASK1 or p38 protects cells from death. (a) COS cells were transfected with siRNAs and 48 h later they were treated with 100 μg/ml of the various lipoprotein for 1 h. After medium exchange and additional incubation for 3 h at 37°C, caspase assays were performed. Data represent mean (±S.D.) values from two independent experiments, each performed six-fold. (b) COS cells were transfected and treated as described in (a), but cells were incubated with lipoprotein (100 μg/ml) or LA (free acid, 0.3 μM) for 16 h before Western blot with anti-p85 PARP was performed (c) Endothelial cells were pretreated with the p38 inhibitor SB203580 (20 μM) or with solvent (DMSO) alone, and 100 μg/ml lipoproteins were subsequently added. After a 1 h incubation in serum-free medium, the lipoproteins were removed and incubation was continued for 3 h in DMEM/1% FCS at 37°C. Caspase 3/7 activity and cellular ATP were assessed in parallel cultures. The data show that SB203580 protected from both caspase activation and cytotoxicity (n=6), **P<0.005 for inhibitor versus solvent alone)
Discussion
A simple explanation can now be provided why excessive retention of LDL in tissues might become dangerous to cells in the immediate vicinity. It is proposed that one important event is the enzymatic liberation of FFA from within the lipoprotein: when present above a critical threshold concentration, these can trigger programmed cell death. Phospholipids represent one pool from which fatty acids may be generated, for example, by the action of sPLA2, an enzyme that is secreted by macrophages, is present in atherosclerotic lesions, and whose involvement in atherogenesis has been reported.20 However, the bulk of fatty acids in LDL are stored as cholesterylesters. Approximately 1700 cholesterylesters packaged within each LDL molecule are normally shielded from the environment by the overlying apolipoprotein B protein shell.21 Proteolytic nicking of apolipoprotein B renders the cholesterylesters accessible to cholesterol ester hydrolase, a lysosomal enzyme that is also present in atherosclerotic lesions.12 Nicking can occur through the action of many different proteases, of which cathepsins D12 and H11 have been detected in early lesions. Combined treatment of LDL with any of these proteases and cholesterylester hydrolase generates a lipoprotein derivative in vitro with the same salient properties as lesional LDL, one dominant feature being a high content of free cholesterol. These recent discoveries account for an older observation whose potential importance is seldom appreciated. On the basis of histochemical stainings, Kruth22 deduced in 1984 that lesional lipids had a high content of free cholesterol. This contention was borne out by subsequent chemical analyses of lipids isolated from early lesions by Simionescu et al in 198623 and by Chao et al in 1988.24 Further verification came from the work of Seifert et al in 19907 and Steinbrecher and Lougheed in 19929 The latter paper reported absence of large amounts of oxidized lipids in early lesions, a finding that was corroborated by Kuhn et al in 1997.25, 26
For these and other reasons, we are considering that nonoxidized, enzymatic remodeling of LDL by proteases and cholesterylester hydrolase may be a key process for the development and evolution of the atherosclerotic lesion.16 Enzymatic modification may not a priori be detrimental. E-LDL binds CRP to activate complement in a controlled manner that excludes the detrimental terminal sequence.26 At subcytotoxic concentrations, FFA in E-LDL selectively stimulate IL-8 production in endothelial cells,27 which would promote monocyte migration into the lesion. E-LDL is rapidly taken up by macrophages,10 and it is proposed that tissues can ultimately be cleared of stranded LDL by the HDL-dependent reverse transport pathway in the absence of inflammatory events. Enzymatic remodeling of LDL is thought to become dangerous only when the cholesterol clearance machinery is overburdened. Then, macrophages begin to produce inflammatory cytokines including IL-6,28 and complement activation bypasses CRP, proceeding to completion with the generation of proinflammatory C5b-9 complexes.26
The present study discloses another dangerous event. FFA, when liberated in excess, can activate apoptosis signaling kinase 1, leading to p38 MAP kinase activation. As has been shown in other studies, massive p38 phosphorylation can unlock mechanisms leading to the activation of executioner caspases.29 The present results indicate that this is a major path to apoptosis in cells exposed to toxic amounts of FFA. In accord with previous findings,5 exposure of cells to native LDL was found to induce a low level of p38 phosphorylation, but this remained without effect on caspase activity and on cell viability. However, massive phosphorylation occurred when the cells were exposed to E-LDL, overcoming the threshold leading to downstream activation of executioner caspases and PARP cleavage. Fatty acid depletion of E-LDL reduced the p38 phosphorylating effect, which was restored upon reconstitution with purified FFA.
An important role for ASK1 upstream of p38-MAPK surfaced in experiments wherein ASK1 silencing was undertaken with the use of siRNA. This abrogated p38 phosphorylation following exposure of cells to E-LDL or FFA, as well as subsequent PARP cleavage.
To test whether oxidation of LDL might be responsible for the observed effects, we compared p38 phosphorylation following exposure of cells to maximally oxidized LDL and to E-LDL. Rather unexpectedly, it was found that ox-LDL exerted essentially no effect further to that already observed with native LDL. This contrasted very strikingly with the marked p38 activation provoked by E-LDL, which contained no detectable thiobarbiturate-reactive substances (TBARS). Furthermore, oxLDL applied at equivalent concentrations entirely failed to rapidly induce apoptosis. These observations rendered it unlikely that the effects of fatty acids reported herein derived from oxidation of the lipoprotein. Our data need not conflict with earlier reports on the cytotoxic effects of oxLDL on endothelial cells.20 In that study, several-fold higher lipoprotein concentrations were used and cyototoxicity became observable only after days of incubation in a nonphysiological milieu devoid of natural antioxidants. In contrast, the triggering events observed in this study occurred within minutes and apoptosis became apparent within hours. Assuredly, the two phenomena are distinct from one another. Rapid activation of MAPKs by FFA contained in E-LDL, and the pathway leading to apoptosis described, both represent novel findings in atherosclerosis research.
In sum, there may be a rather simple but hitherto unrecognized connection between intimal deposition of LDL and apoptotic death of neighboring cells, resulting when lipid deposits exceed a critical threshold so that toxic concentrations of FFA are generated through the action of local enzymes. Future studies should disclose whether this apoptosis signaling pathway is relevant in other cells within atherosclerotic lesions, and in other pathological situations when cells gain contact with high concentrations of FFA. The possibility of rescuing cells through blockade of the apoptosis signaling pathway is of distinct interest and may also have potential therapeutic implications
Materials and Methods
Chemicals
LA, or OA, in water soluble complex with methyl-β-cyclodextrin were obtained from Sigma, sorbitol from Serva. Arachidonic acid and p38-inhibitor SB20358030 were purchased from Calbiochem.
Antibodies
Phospho-p38 MAP kinase (Thr180/Tyr182) rabbit polyclonal antibody, p38 MAP kinase rabbit polyclonal antibody, and HRP-conjugates of anti-biotin, or anti-rabbit Ig were from New England Biolabs. ASK1-F9-mouse monoclonal IgG1 and anti-mouse Ig conjugated to HRP were obtained from Santa Cruz Biotechnology Inc. The production of anti-phospho ASK1 was described previously.31 A polyclonal rabbit antibody specific for the 85 kDa cleavage product of PARP was obtained from Promega, a mouse monoclonal antibody against GAPDH from AMBION.
Isolation and modification of LDL
LDL was isolated and modified as described28 and stored in the presence of EDTA (0.5 mmol/l) for a maximum of 3 weeks. The preparations contained no detectable amounts of TBARS; the TBARS-assay was performed as described by Morel et al32 Oxidation of LDL was achieved by the addition of CuSO4 (25 μmol final concentration) to LDL preparations from which EDTA had been removed by size exclusion chromatography employing PD-10 columns; the mixture was incubated over night at 37°C and CuSO4 was removed again with PD-10 columns. The concentration of malondialdehyde equivalents as measured with the TBAR-assay was ca 0.1 nmol/mg of cholesterol for both native LDL and E-LDL, whereas ox-LDL contained about 7 nmol/mg cholesterol; these values closely match published data. Enzymatic modification of LDL was achieved by sequential incubation with plasmin and cholesterylesterase.13 The content of FFA was reduced as reported earlier.27 In brief, E-LDL was mixed with FFA-free albumin and the mixture was then subjected to ultracentrifugation to recover the lipoprotein by flotation. The resulting lipoprotein preparation is termed E-LDL(d). Incubation with 4% (w/v) albumin (E-LDL(d4)) reduced the FFA content by ∼50%; addition of 10% albumin yielded an E-LDL preparation containing only ∼10% of the original amount of FFA (E-LDL(d10)).27 For some experiments FFA were readded to E-LDL(d) in order to reconstitute the concentrations of a specific FFA in E-LDL (e.g. ca 1250 nmol/mg cholesterol for LA); the reconstituted E-LDL(d) is referred to as ‘R E-LDL(d)’. Lipoprotein concentrations stated in this work refer to total cholesterol in all cases.
Cell culture
Primary endothelial cells from human aorta were used for initial experiments; they were obtained from PromoCell (Heidelberg, Germany), and cultured as suggested by the supplier. Since EA.hy926, a human endothelial cell line (a kind gift of CJS Edgell) proved to respond equally to E-LDL (caspase activation, ATP-loss, p38-phosphorylation, rescue by p38 inhibition, TUNEL-assay), they were employed for the experiments reported here, and are referred to as endothelial cells in the text. COS-7 cells were grown in Dulbecco's modified Eagle's medium/Nutrient Mix F12 (1 : 1) with Glutamax I and pirodoxin (Life Technologies, Paisley, Scotland) supplemented with 10% fetal calf serum (Life Technologies), 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies) at 37°C in humidified air containing 5% CO2.
siRNA-mediated gene silencing
In total, 100 nM each of chemically synthesized siRNA (Ambion) targeting two different regions of the ASK1 gene (sequences available upon request), or a negative control oligonucleotide were pretested by cotransfection with FLAG-ASK1-pcDNA3.0, encoding ASK1-cDNA with an N-terminal FLAG-tag into COS7 cells (3 × 105 cells per well in six-well-plates) for their capacity to inhibit expression of ASK1; transfection was performed with lipofectin in serum-free OptiMEM™. The effect was monitored by Western blot.
For analysis of p38 phosphorylation or caspase assays following ASK1 knockdown, a mixture of the two siRNA targeting ASK1 (100 nM total siRNA concentration), or an equal amount of control siRNA were transfected with 1 μl lipofectin into COS7-cells. Medium was changed 24 h later. Treatment with agents as indicated in figure legends was carried out 48 h post-transfection; cells were harvested by in situ lysis with SDS-loading buffer.
Caspase assay
The Caspase-Glo™ 3/7-reagent from Promega was employed. In total, 2 × 104 cells were seeded per well in 96-well plates 1 day prior to treatment. Lysis and substrate addition were carried out in a one-step procedure. Luminescence was measured using a Berthold Lumat LB 9507 instrument.
Staining with EGFP-Annexin V
Staining of phosphatidylserine in the outer leaflet of apoptotic cells was performed with the Apo-alert-AnnexinV-kit from BD. In total, 5 × 104 cells were seeded in eight-well chamber slides and treated with lipoprotein preparations (200 μg/ml). Cells were stained with Annexin-V without prior fixation according to the manufacturer's protocol. Then the cells were fixed in 2% formaldehyde and stained with DAPI (4,6 Diamidino-2-Phenylindole) (Sigma), 1 μg/ml. Images were taken with an Axiovert 200 microscope connected to a CCD-camera.
Fluorometric DNA-fragmentation assay
A classic TUNEL assay was performed by employing the DeadEnd™ Fluorometric TUNEL System kit of Promega. Endothelial cells (5 × 106) were seeded in chamberslides, treated with 75 mg/ml E-LDL, oxLDL, LDL or LA (free acid) at 0.3 μM, and the assay was performed as detailed in the manufacturer's protocol. Digital images were recorded as given above for the Annexin-stain.
Measurements of cellular ATP
Measurement of intracellular ATP as a parameter of cell viability was performed as described previously28 using the ATP Bioluminescence Assay Kit CLS II from Roche.
Western blot
In total, 25 μl of total cell lysate (corresponding to 1.25 × 105 cells) were separated on a 10% SDS-PAGE, blotted in a semidry-blot chamber onto nitrocellulose membranes. After blocking with 5% skim-milk (or 5% BSA for PARP p85 Western blots) dissolved in TBST for 1 h at RT, membranes were incubated overnight in 5% BSA/TBST containing the primary antibody. Subsequently, membranes were washed three times in TBST and incubated with secondary antibody for 1 h at RT in 0.5% skim milk/TBST. After three washes, blots were developed using BM chemiluminescence Blotting Substrate (POD) from Roche, and autoradiographs were taken. Total p38 and phospho-p38 were sequentially detected on the same Western blot membranes. Stripping of membranes between sequential stains was achieved by 30-min incubation at 50°C in TBS containing 2% SDS and 100 mM β-mercapto-ethanol, followed by two washes in TBST and blocking of the membrane. All Western blot data shown are representative of the available data set of at least two independent experiments per Figure.
Statistics
Data presented in Figures 1a, d, e and 6c are derived from 3–6 independent experiments each, and are given as means±S.D. Statistical significance of the results shown in Figure 6 was determined with an ANOVA. P<0.05 was considered significant.
Abbreviations
- ASK1:
-
apoptosis signal-regulating kinase 1
- CRP:
-
C-reactive protein
- E-LDL:
-
enzymatically modified low-density lipoprotein
- FFA:
-
free fatty acids
- LDL:
-
low-density lipoprotein
- oxLDL:
-
oxidized low-density lipoprotein
- PCD:
-
programmed cell death
- TBARS:
-
thiobarbiturate-reactive substances
References
Martinet W and Kockx MM (2004) Apoptosis in atheroclerosis: implications for plaque destabilization. Verh. K. Acad. Geneeskd. Belg. 66: 61–79
Stoneman VE and Bennett MR (2004) Role of apoptosis in atherosclerosis and its therapeutic implications. Clin. Sci. (London) 107: 343–354
Dimmeler S, Haendeler J and Zeiher AM (2002) Regulation of endothelial cell apoptosis in atherothrombosis. Curr. Opin. Lipidol. 13: 531–536
Artwohl M, Roden M, Waldhausl W, Freudenthaler A and Baumgartner-Parzer SM (2004) Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 18: 146–148
Zhu Y, Liao H, Wang N, Ma KS, Verna LK, Shyy JY, Chien S and Stemerman MB (2001) LDL-activated p38 in endothelial cells is mediated by Ras. Arterioscler. Thromb. Vasc. Biol. 21: 1159–1164
Kruth HS (1985) Cholesterol accumulation in vascular smooth muscle cells incorporated into platelet-rich plasma clots. Lab. Invest. 53: 634–638
Seifert PS, Hugo F, Tranum-Jensen J, Zahringer U and Muhly M andBhakdi S (1990) Isolation and characterization of a complement-activating lipid extracted from human atherosclerotic lesions. J. Exp. Med. 172: 547–557
Fowler SD, Mayer EP and Greenspan P (1985) Foam cells and atherogenesis. Ann. NY Acad. Sci. 454: 79–90
Steinbrecher UP and Lougheed M (1992) Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler. Thromb. 12: 608–625
Bhakdi S, Dorweiler B, Kirchmann R, Torzewski J, Weise E, Tranum-Jensen J, Walev I and Wieland E (1995) On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety. J. Exp. Med. 182: 1959–1971
Han SR, Momeni A, Strach K, Suriyaphol P, Fenske D, Paprotka K, Hashimoto SI, Torzewski M, Bhakdi S and Husmann M (2003) Enzymatically modified LDL induces cathepsin H in human monocytes: potential relevance in early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 23: 661–667
Hakala JK, Oksjoki R, Laine P, Du H, Grabowski GA, Kovanen PT and Pentikainen MO (2003) Lysosomal enzymes are released from cultured human macrophages, hydrolyze LDL in vitro, and are present extracellularly in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 23: 1430–1436
Torzewski M, Suriyaphol P, Paprotka K, Spath L, Ochsenhirt V, Schmitt A, Han SR, Husmann M, Gerl VB, Bhakdi S and Lackner KJ (2004) Enzymatic modification of low-density lipoprotein in the arterial wall: a new role for plasmin and matrix metalloproteinases in atherogenesis. Arterioscler. Thromb. Vasc. Biol. 24: 2130–2136
Torzewski M, Klouche M, Hock J, Messner M, Dorweiler B, Torzewski J, Gabbert HE and Bhakdi S (1998) Immunohistochemical demonstration of enzymatically modified human LDL and its colocalization with the terminal complement complex in the early atherosclerotic lesion. Arterioscler. Thromb. Vasc. Biol. 18: 369–378
Bhakdi S, Torzewski M, Klouche M and Hemmes M (1999) Complement and atherogenesis: binding of CRP to degraded, nonoxidized LDL enhances complement activation. Arterioscler. Thromb. Vasc. Biol. 19: 2348–2354
Bhakdi S, Lackner KJ, Han SR, Torzewski M and Husmann M (2004) Beyond cholesterol: the enigma of atherosclerosis revisited. Thromb. Haemost. 91: 639–645
Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E and Hagiwara M (1996) A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J. Biol. Chem. 271: 13675–13679
Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K and Noda T andIchijo H (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2: 222–228
Takeda K, Matsuzawa A and Nishitoh H andIchijo H (2003) Roles of MAPKKK ASK1 in stress-induced cell death. Cell. Struct. Funct. 28: 23–29
Murakami M and Kudo I (2003) New phospholipase A(2) isozymes with a potential role in atherosclerosis. Curr. Opin. Lipidol. 14: 431–436
Baumstark MW, Kreutz W, Berg A and Keul J (1991) Symmetry of the surface, and structure of the central core of human LDL particles, analyzed by X-ray small angle scattering. Adv. Exp. Med. Biol. 285: 123–130
Kruth HS (1984) Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am. J. Pathol. 114: 201–208
Simionescu N, Vasile E, Lupu F, Popescu G and Simionescu M (1986) Prelesional events in atherogenesis. Accumulation of extracellular cholesterol-rich liposomes in the arterial intima and cardiac valves of the hyperlipidemic rabbit. Am. J. Pathol. 123: 109–125
Chao FF, Amende LM, Blanchette-Mackie EJ, Skarlatos SI, Gamble W, Resau JH, Mergner WT and Kruth HS (1988) Unesterified cholesterol-rich lipid particles in atherosclerotic lesions of human and rabbit aortas. Am. J. Pathol. 131: 73–83
Kuhn H, Heydeck D, Hugou I and Gniwotta C (1997) In vivo action of 15-lipoxygenase in early stages of human atherogenesis. J. Clin. Invest. 99: 888–893
Bhakdi S, Torzewski M, Paprotka K, Schmitt S, Barsoom H, Suriyaphol P, Han SR, Lackner KJ and Husmann M (2004) Possible protective role for C-reactive protein in atherogenesis: complement activation by modified lipoproteins halts before detrimental terminal sequence. Circulation 109: 1870–1876
Suriyaphol P, Fenske D, Zahringer U, Han SR, Bhakdi S and Husmann M (2002) Enzymatically modified nonoxidized low-density lipoprotein induces interleukin-8 in human endothelial cells: role of free fatty acids. Circulation 106: 2581–2587
Klouche M, Gottschling S, Gerl V, Hell W, Husmann M, Dorweiler B, Messner M and Bhakdi S (1998) Atherogenic properties of enzymatically degraded LDL: selective induction of MCP-1 and cytotoxic effects on human macrophages. Arterioscler. Thromb. Vasc. Biol. 18: 1376–1385
Grethe S, Ares MP, Andersson T and Porn-Ares MI (2004) p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp. Cell. Res. 298: 632–642
Zhao M, Liu Y, Wang X, New L, Han J and Brunk UT (2002) Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. Apmis 110: 458–468
Tobiume K, Saitoh M and Ichijo H (2002) Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J. Cell. Physiol. 191: 95–104
Morel DW, Hessler JR and Chisolm GM (1983) Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J. Lipid. Res. 24: 1070–1076
Acknowledgements
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (DFG), Bh2/3-3 to S Bhakdi and M Husmann.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by P Nicotera
Rights and permissions
About this article
Cite this article
Dersch, K., Ichijo, H., Bhakdi, S. et al. Fatty acids liberated from low-density lipoprotein trigger endothelial apoptosis via mitogen-activated protein kinases. Cell Death Differ 12, 1107–1114 (2005). https://doi.org/10.1038/sj.cdd.4401633
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401633
Keywords
This article is cited by
-
Pro-autophagic signal induction by bacterial pore-forming toxins
Medical Microbiology and Immunology (2010)
-
Effects of insulin and free fatty acids on matrix metalloproteinases
Current Diabetes Reports (2008)