Abstract
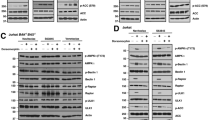
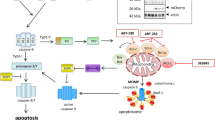
Chemotherapeutic agents and γ-irradiation used in the treatment of brain tumors, the most common solid tumors of childhood, have been shown to act primarily by inducing apoptosis. Here, we report that activation of the CD95 pathway was involved in drug- and γ-irradiation-induced apoptosis of medulloblastoma and glioblastoma cells. Upon treatment CD95 ligand (CD95-L) was induced that stimulated the CD95 pathway by crosslinking CD95 via an autocrine/paracrine loop. Blocking CD95-L/receptor interaction using F(ab′)2 anti-CD95 antibody fragments strongly reduced apoptosis. Apoptosis depended on activation of caspases (interleukin 1β-converting enzyme/Ced-3 like proteases) as it was almost completely abrograted by the broad range caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone. Apoptosis was mediated by cleavage of the receptor proximal caspase FLICE/MACH (caspase-8) and the downstream caspase CPP32 (caspase-3, Apopain) resulting in cleavage of the prototype caspase substrate PARP. Moreover, CD95 was upregulated in wild-type p53 cells thereby increasing responsiveness towards CD95 triggering. Since activation of the CD95 system upon treatment was also found in primary medulloblastoma cells ex vivo, these findings may have implications to define chemosensitivity and to develop novel therapeutic strategies in the management of malignant brain tumors.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by M. Piacentini
Rights and permissions
About this article
Cite this article
Fulda, S., Scaffidi, C., Pietsch, T. et al. Activation of the CD95 (APO-1/Fas) pathway in drug- and γ-irradiation-induced apoptosis of brain tumor cells. Cell Death Differ 5, 884–893 (1998). https://doi.org/10.1038/sj.cdd.4400419
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400419
Keywords
This article is cited by
-
CD95/Fas ligand mRNA is toxic to cells through more than one mechanism
Molecular Biomedicine (2023)
-
Immunological impact of cell death signaling driven by radiation on the tumor microenvironment
Nature Immunology (2020)
-
Heat shock protein inhibitors increase the efficacy of measles virotherapy
Gene Therapy (2008)
-
Paclitaxel (Taxol®)-induced apoptosis in human epithelioid sarcoma cell lines is enhanced by upregulation of CD95 ligand (FasL/Apo-1L)
Journal of Cancer Research and Clinical Oncology (2008)
-
Vincristine and lomustine induce apoptosis and p21WAF1 up-regulation in medulloblastoma and normal human epithelial and fibroblast cells
Journal of Neuro-Oncology (2008)