Abstract
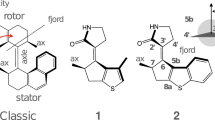
Attempts to fabricate mechanical devices on the molecular level1,2 have yielded analogues of rotors3, gears4, switches5, shuttles6,7, turnstiles8 and ratchets9. Molecular motors, however, have not yet been made, even though they are common in biological systems10. Rotary motion as such has been induced in interlocked systems11,12,13 and directly visualized for single molecules14, but the controlled conversion of energy into unidirectional rotary motion has remained difficult to achieve. Here we report repetitive, monodirectional rotation around a central carbon–carbon double bond in a chiral, helical alkene, with each 360° rotation involving four discrete isomerization steps activated by ultraviolet light or a change in the temperature of the system. We find that axial chirality and the presence of two chiral centres are essential for the observed monodirectional behaviour of the molecular motor. Two light-induced cis-trans isomerizations are each associated with a 180° rotation around the carbon–carbon double bond and are each followed by thermally controlled helicity inversions, which effectively block reverse rotation and thus ensure that the four individual steps add up to one full rotation in one direction only. As the energy barriers of the helicity inversion steps can be adjusted by structural modifications, chiral alkenes based on our system may find use as basic components for ‘molecular machinery’ driven by light.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Feynman,R. P. in Miniaturization (ed. Gilbert, H. D.) (Reinhold, New York, 1961).
Drexler,K. E. Nanosystems: Molecular Machinery, Manufacturing and Computation (Wiley, New York, 1992).
Schoevaars,A. M. et al. Toward a switchable molecular rotor. J. Org. Chem. 62, 4943–4948 (1997).
Clayden,J. & Pink,J. H. Concerted rotation in a tertiary aromatic amide: towards a simple molecular gear. Angew. Chem. Int. Edn Engl. 37, 1937–1939 (1998).
Huck,N. P. M., Jager,W. F., de Lange,B. & Feringa,B. L. Dynamic control and amplification of molecular chirality by circularly polarized light. Science 273, 1686–1688 (1996).
Bissell,S. A., Córdova,E., Kaifer,A. E. & Stoddart,J. F. A chemically and electrochemically switchable molecular shuttle. Nature 369, 133–136 (1994).
Ashton,P. R. et al. Acid-base controllable molecular shuttles. J. Am. Chem. Soc. 120, 11932–11942 (1998).
Bedard,T. C. & Moore,J. S. Design and synthesis of a “molecular turnstile”. J. Am. Chem. Soc. 117, 10662–10671 (1995).
Kelly,T. R., Tellitu,I. & Sestelo,J. P. In search of molecular ratchets. Angew. Chem. Int. Edn Engl. 36, 1866–1868 (1997).
Noji,H., Yasuda,R., Yoshida,M. & Kinosita, K. Jr Directed observation of the rotation of F1-ATPase. Nature 386, 299–302 (1997).
Balzani,V., Gómez-López,M. & Stoddard,J. F. Molecular machines. Acc. Chem. Res. 31, 405–414 (1998).
Sauvage, J.-P. Transition metal-containing rotaxanes and catenanes in motion: toward molecular machines and motors. Acc. Chem. Res. 31, 611–619 (1998).
Armaroli,N. et al. Rotaxanes incorporating two different coordinating units in their thread: synthesis and electrochemically and photochemically induced molecular motions. J. Am. Chem. Soc. 121, 4397–4408 (1999).
Gimzewski,J. K. et al. Rotation of a single molecule within a supramolecular bearing. Science 281, 531–533 (1998).
Harada,N., Koumura,N. & Feringa,B. L. Chemistry of unique chiral olefins. 3. Synthesis and absolute stereochemistry of trans- and cis-1,1′,2,2′,3,3′,4,4′-octahydro-3,3′-dimethyl-4,4′-biphenanthrylidenes. J. Am. Chem. Soc. 119, 7256–7264 (1997).
Koumura,N. & Harada,N. Photochemistry and absolute stereochemistry of unique chiral olefins, trans- and cis-1,1′,2,2′,3,3′,4,4′-octahydro-3,3′-dimethyl-4,4′-biphenanthrylidenes. Chem. Lett. 1151–1152 (1998).
Davis,A. P. Tilting at windmills? The second law survives. Angew. Chem. Int Edn Engl. 37, 909–910 (1998).
Acknowledgements
Financial support from the Netherlands Organisation for Scientific Research (NWO-STW) to B.L.F. is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koumura, N., Zijlstra, R., van Delden, R. et al. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999). https://doi.org/10.1038/43646
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/43646
This article is cited by
-
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Nature Communications (2024)
-
Photo-responsive functional materials based on light-driven molecular motors
Light: Science & Applications (2024)
-
STM studies for surface-mounted molecular rotors: a mini review
AAPPS Bulletin (2024)
-
An electric molecular motor
Nature (2023)
-
Adsorbate motors for unidirectional translation and transport
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.