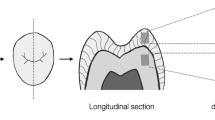
Seamless fixing of an early caries lesion can be achieved without prior excavation.
Abstract
The conventional treatment of dental caries involves mechanical removal of the affected part and filling of the hole with a resin or metal alloy1,2,3,4. But this method is not ideal for tiny early lesions5,6 because a disproportionate amount of healthy tooth must be removed to make the alloy or resin stick. Here we describe a dental paste of synthetic enamel that rapidly and seamlessly repairs early caries lesions by nanocrystalline growth, with minimal wastage of the natural enamel.
Similar content being viewed by others
Main
The human tooth is protected by enamel of 1–2 mm thickness that is composed of hydroxyapatite (Ca10(PO4)6(OH)2) crystals. In early caries lesions, acid-forming bacteria cause microscopic damage to the enamel, creating cavities that are less than 50 µm deep. Such cavities cannot be repaired by simple setting of restorative materials because these do not adhere perfectly to the enamel owing to differences in chemical composition and crystal structure.
We prepared a white crystalline paste of modified hydroxyapatite, which chemically and structurally resembles natural enamel, and used it to repair an early caries lesion in a lower premolar tooth (for methods, see supplementary information).
The affected site was sealed off within 15 min. Examination of the microstructure of the restoration using transmission electron microscopy (TEM) reveals no obvious structural gap at the interface between the regrown layer and the enamel region (Fig. 1a). The regrown layer contains elongated crystals (100–400 nm long and 20–80 nm wide) that have grown across the interface and are regularly orientated to the tooth surface. This shows that the paste has properly integrated with the tooth enamel.
a–c, Transmission electron micrographs, and d–f, atomic-force microscopy images of tooth repair. a, Image of the interface between the regrown layer (upper region) and the enamel (lower region); the arrow indicates the direction of the tooth surface. b, Atomic image of a grown crystal of synthetic enamel. c, Tooth treated with acidic phosphate fluoride; dotted line indicates the interface between the calcium fluoride layer (CF) and enamel (E). d, Original enamel surface; arrow, hydroxyapatite crystal. e, Surface after 3 min of repair time; arrow, newly grown hydroxyapatite crystal. f, Surface after completion of repair (15 min). Scale bars: a, c, f, 100 nm; b, 1 nm; d, e, 50 nm.
An atomic-resolution TEM image of a grown crystal (Fig. 1b) reveals that it has two lattice periodicities that are consistent with the inter-lattice distances for the c and a directions of a hydroxyapatite crystal (0.688 and 0.817 nm, respectively). From these results, combined with those from X-ray photoelectron spectroscopy analysis (data not shown), we conclude that the hydroxyapatite crystals in the regrown layer are oriented with their (0001) face parallel to the tooth surface7. The regrown layer contains about 1% by atoms of fluoride ions and has a calcium-to-phosphorus molar ratio of 1.58±0.03. It also has high durability and acid tolerance (see supplementary information).
For comparison, we repaired a similar lesion with acidic phosphate fluoride solution, an alternative treatment for early caries lesions that does not necessitate the removal of healthy tooth enamel, and examined the restoration by TEM (Fig. 1c). The image shows the presence of a calcium fluoride layer of inhomogeneous thickness8,9 (and less than 1 μm thick) covering the enamel, and a clear gap at the interface (Fig. 1c, dotted line).
Time-lapse atomic-force microscopy indicates that the hydroxyapatite crystals of the original tooth enamel (Fig. 1d) initially dissolved slightly during the repair, but quickly grew again because the paste was acting as a source of crystals. This dissolution and regrowth occurs as a result of the strong acidity (pH<2) of the mother solution and paste. The process creates a continuous, nanometre-scale structure that extends from the enamel to the regrown layer by epitaxial growth of crystals.
The newly grown crystals of hydroxyapatite cover the whole surface in a densely packed array after 3 min (Fig. 1e), and are three-dimensionally stacked after 15 min (Fig. 1f). The acidic conditions probably contribute to the fast growth of highly crystalline hydroxyapatite by dissociating the calcium phosphate clusters into calcium and phosphate ions10,11; the clusters would otherwise slow growth rates and cause low crystallinity.
We have shown that our synthetic material can reconstruct enamel without prior excavation, in a process that not only repairs early caries lesions but can also help to prevent their reoccurrence by strengthening the natural enamel. In the clinic, the paste should not come into contact with the gums, where its acidity and its high concentration of hydrogen peroxide could cause inflammation (though materials with similarly adverse properties are already used on patients12).
References
Raskin, A., Michotte-Theall, B., Vreven, J. & Wilson, N. H. F. J. Dent. 27, 13–19 (1999).
Wilson, N. H. F. & Mjor, I. A. J. Dent. 28, 15–21 (2000).
Carvalho, R. M., Pereira, J. C., Yoshiyama, M. & Pashley, D. H. Oper. Dent. 21, 17–24 (1996).
Hilton, T. J. Am. J. Dent. 15, 198–210 (2002).
Frank, R. M. & Brendel, A. Archs Oral Biol. 11, 883–912 (1966).
Johnson, N. W. Caries Res. 1, 356–369 (1967).
Elliot, J. C. (ed) Structure and Chemistry of the Apatites and Other Calcium Orthophosphates (Elsevier, Amsterdam, 1994).
Gerould, H. J. Dent. Res. 24, 223–233 (1945).
Duschner, H., Gotz, H. & Ogaard, B. Eur. J. Oral Sci. 105, 466–472 (1997).
Onuma, K. & Ito, A. Chem. Mater. 10, 3346–3351 (1998).
Banfield, J. F., Welch, S. A., Zhang, H., Ebert, T. T. & Penn, R. L. Science 289, 751–754 (2000).
Yamagishi, K. & Suzuki, T. J. Esthetic Dent. 7, 78–80 (1995).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
The file is composed of two figure panels (a-b) that show an example of a tooth (extracted upper premolar) before (a) and after (b) repair using our paste. (JPG 41 kb)
Supplementary Figure 2a-f
The three files contain eight panels (a-h) showing laser confocal microscopy images of the dissolution process of a paste-repaired surface (a-c) and dental enamel (d-f) in acidic simulated saliva, plus dissolution rate measurements (g), and an SEM photograph of a paste-repaired tooth after a 10,000 times brushing test. (JPG 230 kb)
Supplementary Methods
The document describes the preparation of our paste and repair protocol for using the paste. The document also describes the chemical composition of APF solution and the conditions for the durability and acid tolerance tests as well as the chemical composition of simulated saliva. (DOC 21 kb)
Rights and permissions
About this article
Cite this article
Yamagishi, K., Onuma, K., Suzuki, T. et al. A synthetic enamel for rapid tooth repair. Nature 433, 819 (2005). https://doi.org/10.1038/433819a
Published:
Issue Date:
DOI: https://doi.org/10.1038/433819a
This article is cited by
-
High-resolution Raman spectroscopy reveals compositional differences between pigmented incisor enamel and unpigmented molar enamel in Rattus norvegicus
Scientific Reports (2023)
-
Tooth whitening with an experimental toothpaste containing hydroxyapatite nanoparticles
BMC Oral Health (2022)
-
Advances in biomineralization-inspired materials for hard tissue repair
International Journal of Oral Science (2021)
-
Restorative dental resin functionalized with methacryloxy propyl trimethoxy silane to induce reversible in situ generation of enamel-like hydroxyapatite
Journal of Materials Science (2018)
-
Protein disorder–order interplay to guide the growth of hierarchical mineralized structures
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.