Abstract
Even though the genetic fingerprint of human sperm has been defined, its role in orchestrating fertilization and the development of the early embryo remains vague. Here we show that human male gametes pass over more to the oocyte than just the haploid male genome — paternal messenger RNAs are also delivered to the egg at fertilization. If these transcripts, previously thought to be left-overs from spermatogenesis, are important in early development, our findings may have implications for the success of somatic-cell nuclear transfer in cloning technology and the identification of components leading to unexplained male-factor infertility.
Similar content being viewed by others
Main
Microarray analysis has identified a set of transcripts that are common to both human testes and spermatozoa and are implicated in fertilization as well as in zygotic and embryonic development1. We investigated this possibility using the polymerase chain reaction (PCR) with reverse transcription to determine which of these transcripts is present in human spermatozoa but not in unfertilized human oocytes, and identified six candidates (Table 1). The implication is that spermatozoa deliver these transcripts to the ooplasm at fertilization.
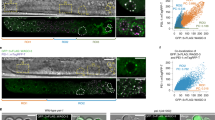
We therefore used the zona-free hamster egg/human sperm penetration assay (a standard clinical assay to test the virility of human sperm) to investigate this possibility (for methods, see supplementary information). Complementary DNAs from human spermatozoa, hamster oocytes and hybrid human–hamster zygotes were interrogated by real-time PCR; these RNA preparations were devoid of DNA contamination (the 310-base-pair protamine-2 gene was not detected). Only the protamine-2 and clusterin transcripts were consistently detected in spermatozoa, zygotes and in the positive control, and not detected in hamster oocytes or in the negative control (Fig. 1). These results show that spermatozoa deliver RNAs to the oocyte at fertilization.
a, Protamine-2; b, clusterin. All RNAs, including messenger RNAs, were isolated from human spermatozoa, hamster oocytes and zygotes of hamster oocytes fertilized by human spermatozoa at 30 min and 3 h post-fertilization. The transfer of spermatozoa-specific RNA was assayed by real-time polymerase chain reaction (PCR): half of the reaction mixture was used to confirm product size on 2% agarose gels stained with ethidium bromide. PCR fidelity was verified with human genomic DNA and testis complementary DNA (cDNA) as positive controls and hamster genomic DNA and water as negative controls. Lanes in both gels: (1) size-calibration ‘ladder’ (100–1,000 base pairs (bp) at 100-bp increments, plus 1,200- and 1,500-bp standards); (2) human spermatozoa cDNA; (3) hamster oocyte cDNA; (4) zygotic cDNA at 30 min post-fertilization; (5) zygotic cDNA at 3 h post-fertilization; (6) human genomic DNA; (7) hamster genomic DNA; (8) human testis cDNA; (9) water. The protamine-2 sequences (148 bp for cDNA; 310 bp for genomic DNA) or clusterin sequences (110 bp) are evident in spermatozoa, zygotes and the positive control, but not in hamster oocytes or in the negative control.
Why should spermatozoa messenger RNAs be transferred to the oocyte? Messenger RNAs encoding proteins that bind nucleic acids, such as protamine-2, are likely to be deleterious and are probably degraded following entry2, and a similar fate may await other RNAs that gain access3. But some may have a role in the developing zygote: for example, clusterin (also known as sulphated glycoprotein-2, or SGP-2) is delivered to the oocyte and has been implicated in cell–cell and cell–substratum interactions, enhancement of fertility rate, lipid transportation, membrane recycling, stabilization of stress proteins, and promotion or inhibition of apoptosis4,5,6,7. These may therefore be required in the early zygote but unnecessary in the oocyte. Alternatively (or in addition), these and other unidentified molecules, such as small interfering RNAs (siRNAs), may participate in processes such as pronuclear formation, the orchestration of events leading to oocyte activation, the transition from maternal to embryonic gene control, and the establishment of imprints in early embryos.
It could be argued that successful parthenogenesis and somatic-cell nuclear-transfer experiments preclude a role for paternal RNAs in these processes. Parthenogenetic activation has been known to support development up to the limb-bud stage (corresponding to about 30 somites)8,9, and only recently a ‘parthenogenetic’ (derived from two different egg cells) mammal was born after genome modification10. However, the success of such experiments and of somatic-cell nuclear transfer is limited11, as is the production of human embryonic stem cells after somatic-cell nuclear transfer12. This may be because sperm RNAs contribute to early development. Transcripts that are specific to male germ cells play a role in the differentiation of embryonic stem cells13 and their function may not be easily replaced.
Our results indicate that sperm RNAs could be important in early zygotic and embryonic development and that they may hold the key to more successful somatic-cell nuclear transfer or to the identification of male-derived factors that underlie idiopathic infertility.
References
Ostermeier, G. C., Dix, D. J., Miller, D., Khatri, P. & Krawetz, S. A. Lancet 360, 772–777 (2002).
Ziyyat, A. & Lefevre, A. Hum. Reprod. 16, 1449–1456 (2001).
Hayashi, S., Yang, J., Christenson, L., Yanagimachi, R. & Hecht, N. B. Biol. Reprod. 69, 1170–1176 (2003).
O’Bryan, M. K. et al. J. Clin. Invest. 85, 1477–1486 (1990).
Poon, S. et al. J. Biol. Chem. 277, 39532–39540 (2002).
Trougakos, I. P. & Gonos, E. S. Int. J. Biochem. Cell Biol. 34, 1430–1448 (2002).
Wong, P. et al. Eur. J. Biochem. 221, 917–925 (1994).
Kono, T., Sotomaru, Y., Katsuzawa, Y. & Dandolo, L. Dev. Biol. 243, 294–300 (2002).
Kure-Bayashi, S., Miyake, M., Okada, K. & Kato, S. Theriogenology 53, 1105–1119 (2000).
Kono, T. et al. Nature 428, 860–864 (2004).
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J. & Campbell, K. H. Nature 385, 810–813 (1997).
Hwang, W. S. et al. Science 303, 1669–1674 (2004).
Geijsen, N. et al. Nature 427, 148–154 (2004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Methods
Includes information on zona-free hamster penetration assay; RNA extraction; RT-PCR; real time PCR. (DOC 75 kb)
Rights and permissions
About this article
Cite this article
Ostermeier, G., Miller, D., Huntriss, J. et al. Delivering spermatozoan RNA to the oocyte. Nature 429, 154 (2004). https://doi.org/10.1038/429154a
Issue Date:
DOI: https://doi.org/10.1038/429154a
This article is cited by
-
Semen proteome and transcriptome of the endangered black-footed ferret (Mustela nigripes) show association with the environment and fertility outcome
Scientific Reports (2024)
-
Phthalates impact on the epigenetic factors contributed specifically by the father at fertilization
Epigenetics & Chromatin (2023)
-
HA-tag CD63 is a novel conditional transgenic approach to track extracellular vesicle interactions with sperm and their transfer at conception
Scientific Reports (2023)
-
Transcriptome Analysis Reveals Spermatogenesis-Related CircRNAs and LncRNAs in Goat Spermatozoa
Biochemical Genetics (2023)
-
Development and validation of most efficient RNA isolation method from buffalo bull spermatozoa
Molecular Biology Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.