Abstract
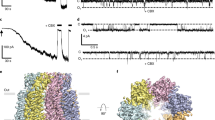
The entry and exit of water from cells is a fundamental process of life. Recognition of the high water permeability of red blood cells led to the proposal that specialized water pores exist in the plasma membrane1. Expression in Xenopus oocytes and functional studies of an erythrocyte integral membrane protein of relative molecular mass 28,000, identified it as the mercury-sensitive water channel, aquaporin-1 (AQP1)2. Many related proteins, all belonging to the major intrinsic protein (MIP) family, are found throughout nature3. AQP1 is a homotetramer containing four independent aqueous channels4,5,6. When reconstituted into lipid bilayers, the protein forms two-dimensional lattices with a unit cell containing two tetramers in opposite orientation7,8,9,10. Here we present the three-dimensional structure of AQP1 determined at 6Å resolution by cryo-electron microscopy. Each AQP1 monomer has six tilted, bilayer-spanning α-helices which form a right-handed bundle surrounding a central density. These results, together with functional studies, provide a model that identifies the aqueous pore in the AQP1 molecule and indicates the organization of the tetrameric complex in the membrane.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sidel, V. W. & Solomon, A. K. Entrance of water into human red cells under an osmotic pressure gradient. J. Gen. Physiol. 41, 243–257 (1957).
Preston, G. M., Carroll, T. P., Guggino, W. B. & Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256, 385–387 (1992).
Park, J. H. & Saier, M. H. Phylogenetic characterization of the MIP family of transmembrane channel proteins. J. Memb. Biol. 153, 171–180 (1996).
Smith, B. L. & Agre, P. Erythrocyte Mr28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J. Biol. Chem. 266, 6407–6415 (1991).
Verbavatz, J.-M. et al. Tetrameric assembly of CHIP28 water channels in liposomes and cell membranes: a freeze-fracture study. J. Cell Biol. 123, 605–618 (1993).
Jung, J. S., Preston, G. M., Smith, B. L., Guggino, W. B. & Agre, P. Molecular structure of the water channel through Aquaporin CHIP: the tetrameric-hourglass model. J. Biol. Chem. 269, 14648–14654 (1994).
Walz, T., Smith, B. L., Agre, P. & Engel, A. The three-dimensional structure of human erythrocyte aquaporin CHIP. EMBO J. 13, 2985–2993 (1994).
Walz, T., Typke, D., Smith, B. L., Agre, P. & Engel, A. Projection map of aquaporin-1 determined by electron crystallography. Nature Struct. Biol. 2, 730–732 (1995).
Mitra, A. K., van Hoek, A. N., Wiener, M. C., Verkman, A. S. & Yeager, M. The CHIP28 water channel visualized in ice by electron crystallography. Nature Struct. Biol. 2, 726–729 (1995).
Jap, B. K. & Li, H. Structure of the osmo-regulated H2O-channel, AQP-CHIP, in projection at 3.5Å. J. Mol. Biol. 251, 413–420 (1995).
Preston, G. M., Jung, J. S., Guggino, W. B. & Agre, P. Membrane topology of aquaporin CHIP: analysis of functional epitope-scanning mutants by vectorial proteolysis. J. Biol. Chem. 269, 1668–1673 (1994).
Walz, T. et al. Surface topographies at subnanometer resolution reveal asymmetry and sidedness of aquaporin-1. J. Mol. Biol. 264, 907–918 (1996).
Cabiaux, V. et al. Comparison of aquaporin-1 and bacteriorhodopsin: a Fourier-transform infrared spectroscopy study. Biophysical J.(in the press).
Walther, D., Eisenhaber, F. & Argos, P. Principles of helix–helix packing in proteins: The helical lattice superposition model. J. Mol. Biol. 255, 536–553 (1996).
Walz, T., Smith, B. L., Zeidel, M. L., Engel, A. & Agre, P. Biologically active two-dimensional crystals of aquaporin CHIP. J. Biol. Chem. 269, 1583–1586 (1994).
Fujiyoshi, Y. et al. Development of a superfluid helium stage for high-resolution electron microscopy. Ultramicroscopy 38, 241–251 (1991).
Mitsuoka, K., Murata, K., Kimura, A. H., Namba, K. & Fujiyoshi, Y. Examination of the LeafScan 45, a line-illuminating micro-densitometer, for its use in electron crystallography. Ultramicroscopy(in the press).
Crowther, R. A., Henderson, R. & Smith, J. M. MRC image processing programs. J. Struct. Biol. 116, 9–16 (1995).
Henn, C., Teschner, M., Engel, A. & Aebi, U. Real-time isocontouring and texture mapping meet new challenges in interactive molecular graphics applications. J. Struct. Biol. 116, 86–92 (1995).
Henderson, R. et al. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 213, 899–929 (1990).
Acknowledgements
This work has been supported by the Swiss National Foundation for Scientific Research, the Maurice E. Müller Foundation of Switzerland, the State of Basel, the National Institutes of Health, and the Japan Society for the Promotion of Science-Research for the Future Program. T.W. Thanks the EMBO for a fellowship.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walz, T., Hirai, T., Murata, K. et al. The three-dimensional structure of aquaporin-1. Nature 387, 624–627 (1997). https://doi.org/10.1038/42512
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/42512
This article is cited by
-
Aquaporin water channels: roles beyond renal water handling
Nature Reviews Nephrology (2023)
-
Mechanosensitive aquaporins
Biophysical Reviews (2023)
-
Ethanol yield improvement in Saccharomyces cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta mutant and molecular mechanism exploration based on the metabolic flux and transcriptomics approaches
Microbial Cell Factories (2022)
-
A PIP-mediated osmotic stress signaling cascade plays a positive role in the salt tolerance of sugarcane
BMC Plant Biology (2021)
-
Bioinspired and biomimetic membranes for water purification and chemical separation: A review
Frontiers of Environmental Science & Engineering (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.