Abstract
Since the discovery of their one-dimensional electronic band structure1, the leading candidate that has emerged for nanodevice applications is single-walled carbon nanotubes (SWNTs) . Here we unite their unique properties with the specific molecular-recognition features of DNA by coupling SWNTs to peptide nucleic acid (PNA, an uncharged DNA analogue2) and hybridizing these macromolecular wires with complementary DNA. Our findings provide a new, versatile means of incorporating SWNTs into larger electronic devices by recognition-based assembly, and of using SWNTs as probes in biological systems by sequence-specific attachment.
Similar content being viewed by others
Main
At present, SWNT-based devices such as field-effect transistors3, logic circuits4,5 and single-electron transistors6 are fabricated by 'top-down' lithographic methods. The construction of more complex architectures with high device density requires the development of a 'bottom-up', massively parallel strategy that exploits the molecular properties of SWNTs.
We have therefore developed a technique to couple SWNTs covalently to PNA. Our process begins by ultrasonically shortening SWNT ropes (Tubes@Rice) for 1 hour in a 3:1 mixture of concentrated H2SO4 and HNO3. Subsequent exposure to 1 M HCl produces abundant carboxyl end-groups7. This material is dispersed in dimethylformamide (DMF, 99.5%) and incubated for 30 min in 2 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and 5 mM N-hydroxysuccinimide (NHS) to form SWNT-bearing NHS esters8 (Fig. 1a).
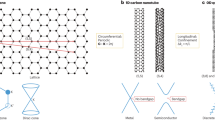
a, b, N-hydroxysuccinimide (NHS) esters formed on carboxylated, single-walled carbon nanotubes (SWNTs) are displaced by peptide nucleic acid (PNA), forming an amide linkage. c, A DNA fragment with a single-stranded, 'sticky' end hybridizes by Watson–Crick base-pairing to the PNA–SWNT. d, e, Atomic-force microscope (TappingMode) images of PNA–SWNTs. SWNTs appear as bright lines; the paler strands represent bound DNA. Scale bars: 100 nm; nanotube diameters: d, 0.9 nm; e, 1.6 nm.
PNA adducts are formed (Fig. 1b) by reacting this material in DMF for 1 hour with excess PNA (sequence: NH2-Glu– GTGCTCATGGTG–CONH2, where Glu is a glutamate amino-acid residue and the central block represents nucleic-acid bases; Fig. 1b). The PNA-derivatized SWNTs are transferred to water and dispersed in 0.5% aqueous sodium dodecyl sulphate, which stabilizes the individual SWNTs9.
To investigate whether DNA would hybridize to PNA–SWNTs, we prepared fragments of double-stranded DNA with 12-base-pair, single-stranded 'sticky' ends that were complementary to the PNA sequence. These fragments were produced by cutting double-stranded DNA with the restriction enzyme HindIII and ligating the products to single-stranded oligonucleotides. This sticky DNA was hybridized to the PNA–SWNTs (Fig. 1c) in water, deposited on freshly cleaved mica with 5 mM MgCl2, and the surface was rinsed and dried after 30 s. Atomic-force micrographs (Fig. 1d, e) of the DNA/PNA–SWNT hybrids were recorded under ambient conditions.
From our observations of several samples, we conclude that DNA attachment occurs predominantly at or near the nanotube ends. Because the derivatization chemistry is done on SWNT 'ropes', we contend that individual SWNTs are largely shielded, resulting in a low density of defects in their side walls. The rare attachment of DNA to other regions of SWNTs also indicates that this is the result of sequence-specific PNA–DNA base-pairing, rather than of nonspecific interaction.
We chose to couple PNA, rather than DNA, directly with SWNTs for several reasons10. First, PNA is compatible with the most convenient solvents (DMF, for example); second, PNA is not susceptible to enzymatic degradation; and third, the uncharged PNA backbone gives rise to PNA–DNA duplexes that are more thermally stable than their DNA–DNA counterparts because there is no electrostatic repulsion. Besides reducing the length of the sticky ends required for room-temperature hybridization, this last property should reduce nonspecific electrostatic interactions with metallic electrodes or with surfaces, such as silicon oxide, that are convenient for lithography.
The recognition properties imparted to SWNTs by oligonucleotide adducts could be used to programme the attachment of SWNTs to each other and to substrate features, such as electrodes, on which monolayers of complementary sequences are self-assembled. The antisense properties of PNA–SWNTs might also be exploited in a biological context, for example in biosensors. Our results represent a step towards full compatibility of SWNTs with enzymes and proteins11,12 — a powerful approach for organizing complex devices at the sublithographic scale.
References
Mintmire, J. W., Dunlap, B. I. & White, C. T. Phys. Rev. Lett. 68, 631–634 (1992).
Nielsen, P. E., Engholm, M., Berg, R. H. & Buchardt, O. Science 254, 1497–1500 (1991).
Tans, S., Verschueren, A. R. M. & Dekker, C. Nature 393, 49–52 (1998).
Derycke, V., Martel, R., Appenzeller, J. & Avouris, P. Nano Lett. 1, 453–456 (2001).
Bachtold, A., Hadley, P., Nakanishi, T. & Dekker, C. Science 294, 1317–1320 (2001).
Postma, H. W. C., Teepen, T. F., Yao, Z., Grifoni, M. & Dekker, C. Science 293, 76–79 (2001).
Chen, J. et al. Science 282, 95–98 (1998).
Grabarek, Z. & Gergely, J. Analyt. Biochem. 185, 131–135 (1990).
O'Connell, M. J. et al. Science 297, 593–596 (2002).
Wang, J. Biosensors Bioelectron. 13, 757–762 (1998).
Wong, S. S., Joselevich, E., Woolley, A. T., Cheung, C. L. & Lieber, C. M. Nature 394, 52–55 (1998).
Huang, W. et al. Nano Lett. 2, 311–314 (2002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Williams, K., Veenhuizen, P., de la Torre, B. et al. Carbon nanotubes with DNA recognition. Nature 420, 761 (2002). https://doi.org/10.1038/420761a
Issue Date:
DOI: https://doi.org/10.1038/420761a
This article is cited by
-
The interaction of nucleobases with an AlN nanotube for electronic DNA sequencing
Journal of Computational Electronics (2021)
-
Nucleic Acid Nanoprobes for Biosensor Development in Complex Matrices
Chemical Research in Chinese Universities (2020)
-
Targeting autophagy using metallic nanoparticles: a promising strategy for cancer treatment
Cellular and Molecular Life Sciences (2019)
-
Biomedical applications of nanotechnology
Biophysical Reviews (2017)
-
Adsorption of DNA binding proteins to functionalized carbon nanotube surfaces with and without DNA wrapping
European Biophysics Journal (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.