Even immature oocytes can eventually be fertilized after some skilful manipulation.
Abstract
Nuclear reprogramming is essential during gametogenesis for the production of totipotent zygotes. Here we show that premeiotic female germ cells derived from mouse fetuses as early as 12.5 days post coitum are able to complete meiosis and genomic imprinting in vitro and that these matured oocytes are highly competent in supporting development to full term after nuclear transfer and in vitro fertilization. To our knowledge, this is the first time that complete oogenesis has been successfully accomplished in vitro.
Similar content being viewed by others
Main
Although the ovaries of mammals contain thousands or millions of immature oocytes, few of these ever mature to the point at which reproduction in vivo is possible. Ovarian oocytes therefore constitute a large and potentially valuable resource for clinical and zoological application. However, although mature oocytes have been produced in vitro by culturing immature oocytes1,2,3, oogenesis was never complete, and attempts to produce even non-growing oocytes at the diplotene stage of the first meiosis from ovaries derived from newborn mice have met with limited success (only 0.016%; ref. 2).
We have shown that this poor ability of oocytes to mature in culture is due to their incompetent cytoplasm4, a problem that can be overcome by transferring their nuclei into enucleated, fully grown oocytes. Although live pups can eventually be produced from oocytes reconstituted in this way, this is not possible if nuclei from small, immature oocytes are used for reconstitution, probably because of defects in their meiotic chromosomal configuration and/or genomic imprinting5,6,7.
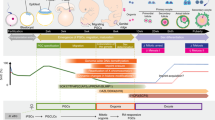
We cultured premeiotic female germ cells in vitro in an attempt to complete this essential nuclear reprogramming (see supplementary information for details). Ovaries from mouse fetuses at 12.5 days post coitum (d.p.c.), with the mesonephroi attached, were cultured for 7 days, followed by removal of the mesonephroi and a further 10 days in culture (Fig. 1). These cultured ovaries contained many secondary follicles, which we isolated and cultured for 11 days; at the end of the 28-day culture period, some follicles showed antrum formation and the oocytes had increased in diameter (63.9 µm, n = 127; Fig. 1).
Day 0, ovary (ov) from a green-fluorescent-protein (GFP)-labelled B6CBF1 (GFP–C57BL/6J × CBA) transgenic mouse fetus at 12.5 d.p.c.11, with attached mesonephroi (m). Ovaries were cultured in Waymouth medium supplemented with 10% fetal bovine serum (FBS) on a Costar Transwell membrane2. At day 7 the mesonephroi was removed and at day 17 a secondary follicle, consisting of theca cells (t) and 2–3 layers of granulosa cells (g) around the oocyte (o), was isolated from the ovary and cultured in MEM-α medium supplemented with 5% FBS, 0.1 IU ml−1 follicle-stimulating hormone, 5 µg ml−1 insulin, 5 µg ml−1 transferrin and 5 ng ml−1 selenium on a Costar Transwell-COL membrane2,3. Day 28, oocyte isolated from cultured follicle for nuclear transfer. After nuclear transfer and in vitro fertilization, the resulting zygotes (bottom right. day 49) were cultured in M16 medium. All cultures were incubated at 37 °C in an atmosphere of 5% CO2 in air. Scale bars: white, 500 µm; black, 50 µm.
We estimated the extent of genomic imprinting in these oocytes by analysing DNA methylation in the imprinted gene Igf2r (ref. 8). The methylation pattern at each stage of culture was consistent with that in oocytes taken from mice at the same stage (see supplementary information), indicating that normal imprinting can be established in vitro.
We also investigated whether nuclear reprogramming could occur in these oocytes by monitoring their development (see supplementary information). Because oocytes isolated from cultured follicles were unable to resume meiosis (0/38, 0%), we transferred the nuclei into enucleated, fully grown oocytes from adult mice. These reconstituted oocytes were able to resume meiosis and mature into metaphase in the second meiosis (M II, 101/108, 94%) and had a normal karyotype (n = 20, 11/11, 100%), indicating that chromosomal maturation for meiosis can be completed in vitro. As expected4, oocytes reconstituted with intact female germ cells from fetuses at 12.5 d.p.c. became aneuploid (32/32, 100%) owing to abnormal chromosomal segregation.
As oocytes reconstituted by a single nuclear transfer did not develop efficiently into blastocysts (14/34, 41%), we used serial nuclear transfer (see supplementary information for details). The rate of in vitro fertilization of these reconstituted oocytes was normal (72/81, 89%) and they developed efficiently into blastocysts (64/72, 89%).
After embryo transfer to a surrogate mother, 16 living pups were obtained from 7 mothers at 19.0 d.p.c. by caesarean section (16/64, 25%). No obvious abnormality was seen in any of the pups (in vitro pup weight compared with that in vivo: 1.46 g versus 1.34 g, P > 0.1) or placentae (in vitro weight compared with that in vivo: 133 mg versus 126 mg, P > 0.5), as expected from normal imprinted methylation of Igf2r, Snrpn and Peg1 (see supplementary information)9,10. These animals were fertile after puberty.
We have shown that the most primitive murine fetal oocytes can differentiate into competent oocytes with high efficiency. As well as offering an opportunity to analyse the mechanisms behind nuclear reprogramming in vitro, our system might eventually help women undergoing chemotherapy or radiotherapy to become mothers afterwards, by prior removal of an ovary.
References
Buehr, M. & McLaren, A. Gamete Res. 11, 271–281 (1985).
Eppig, J. J. & O'Brien, M. J. Biol. Reprod. 54, 197–207 (1996).
Cortvrindt, R., Smitz, J. & Van Steirteghem, A. C. Hum. Reprod. 11, 2656–2666 (1996).
Bao, S., Obata, Y., Carroll, J., Domeki, I. & Kono, T. Biol. Reprod. 62, 616–621 (2000).
Kono, T., Obata, Y., Yoshimzu, T., Nakahara, T. & Carroll, J. Nature Genet. 13, 91–94 (1996).
Obata, Y. et al. Development 125, 1553–1560 (1998).
Obata, Y. & Kono, T. J. Biol. Chem. 277, 5285–5289 (2002).
Wutz, A. et al. Nature 389, 745–749 (1997).
Bourc'his, D., Xu, G. L., Lin, C. S., Bollman, B. & Bestor, T. H. Science 294, 2536–2539 (2001).
Lucifero, D., Mertineit, C., Clarke, H. J., Bestor, T. H. & Trasler, J. M. Genomics 79, 530–538 (2002).
Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. FEBS Lett. 407, 313–319 (1997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Obata, Y., Kono, T. & Hatada, I. Maturation of mouse fetal germ cells in vitro. Nature 418, 497 (2002). https://doi.org/10.1038/418497a
Issue Date:
DOI: https://doi.org/10.1038/418497a
This article is cited by
-
Complete in vitro oogenesis: retrospects and prospects
Cell Death & Differentiation (2017)
-
Development of fertile mouse oocytes from mitotic germ cells in vitro
Nature Protocols (2017)
-
Differentiation of Mouse Primordial Germ Cells into Functional Oocytes In Vitro
Annals of Biomedical Engineering (2017)
-
Etoposide damages female germ cells in the developing ovary
BMC Cancer (2016)
-
In vitro differentiation of germ cells from stem cells: a comparison between primordial germ cells and in vitro derived primordial germ cell-like cells
Cell Death & Disease (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.