Abstract
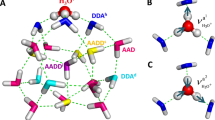
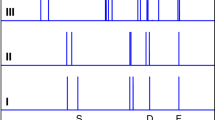
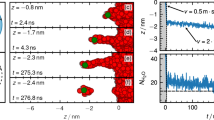
Ionization and dissociation reactions play a fundamental role in aqueous chemistry. A basic and well-understood example is the reaction between hydrogen chloride (HCl) and water to form chloride ions (Cl-) and hydrated protons (H3O+ or H5O2+). This acid ionization process also occurs in small water clusters1,2,3,4 and on ice surfaces5,6,7,8,9,10,11,12,13,14,15,16,17, and recent attention has focused on the mechanism of this reaction in confined-water media and the extent of solvation needed for it to proceed1,2,3,4,9,15,16,17. In fact, the transformation of HCl adsorbed on ice surfaces from a predominantly molecular form to ionic species during heating from 50 to 140 K has been observed8,13,14. But the molecular details of this process remain poorly understood. Here we report infrared transmission spectroscopic signatures of distinct stages in the solvation and ionization of HCl adsorbed on ice nanoparticles kept at progressively higher temperatures. By using Monte Carlo and ab initio simulations to interpret the spectra, we are able to identify slightly stretched HCl molecules, strongly stretched molecules on the verge of ionization, contact ion pairs comprising H3O+ and Cl-, and an ionic surface phase rich in Zundel ions, H5O2+.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Packer, M. J. & Clary, D. C. Interaction of HCl with water clusters: (H2O)nHCl, n = 1-3. J. Phys. Chem. 99, 14323–14333 (1995)
Re, S., Osamura, Y., Suzuki, Y. & Schaefer, H. F. Structures and stability of hydrated clusters of hydrogen chloride, HCl(H2O)n, n = 1-5. J. Chem. Phys. 109, 973–977 (1998)
Bacelo, D. E., Binning, R. C. & Ishikawa, Y. Ab initio Monte Carlo simulated annealing study of HCl(H2O)n (n = 3,4) clusters. J. Phys. Chem. A 103, 4631–4640 (1999)
Chaban, G. M., Gerber, R. B. & Janda, K. C. Transition from hydrogen bonding to ionization in (HCl)n(NH3)n and (HCl)n(H2O)n clusters. J. Phys. Chem. A 105, 8323–8332 (2000)
Hanson, D. R. & Ravishankara, A. R. Investigation of the reactive and non-reactive processes involving ClONO2 and HCl on water and nitric acid doped ice. J. Phys. Chem. 96, 2682–2691 (1992)
Horn, A. B., Chesters, M. A., McCoustra, M. R. S. & Sodeau, J. R. Adsorption of stratospherically important molecules on thin D2O films using reflection absorption infrared spectroscopy. J. Chem. Soc. Faraday Trans. 88, 1077–1078 (1992)
Chu, L. T., Leu, M. T. & Keyser, L. F. Uptake of HCl in water ice and nitric acid ice films. J. Phys. Chem. 97, 7779–7785 (1993)
Delzeit, L., Rowland, B. & Devlin, J. P. Infrared-spectra of HCl complexed/ionized in amorphous hydrates and at ice surfaces in the 15-90 K range. J. Phys. Chem. 97, 10312–10318 (1993)
Robertson, S. H. & Clary, D. C. Solvation of hydrogen halides on the surface of ice. Faraday Discuss. 100, 309–320 (1995)
Banham, S. F., Horn, A. B., Koch, T. G. & Sodeau, J. R. Ionisation and solvation of stratospherically relevant molecules on ice films. Faraday Discuss. 100, 321–332 (1995)
Graham, J. D. & Roberts, J. T. Interaction of HCl with crystalline and amorphous ice—implications for the mechanisms of ice-catalyzed reactions. Geophys. Res. Lett. 22, 251–254 (1995)
Gertner, B. J. & Hynes, J. T. Model molecular dynamics simulation of hydrochloric acid ionization at the surface of stratospheric ice. Faraday Discuss. 110, 301–322 (1998)
Isakson, M. J. & Sitz, G. O. Adsorption and desorption of HCl on ice. J. Phys. Chem. A 103, 2044–2049 (1999)
Kang, H., Shin, T. H., Park, S. C., Kim, I. K. & Han, S. J. Acidity of hydrogen chloride on ice. J. Am. Chem. Soc. 122, 9842–9843 (2000)
Allouche, A., Couturier-Tamburelli, I. & Chiavassa, T. Ab initio model study of the mechanism of hydrogen chloride ionization on ice. J. Phys. Chem. B 104, 1497–1506 (2000)
Svanberg, M., Pettersson, J. B. C. & Bolton, K. J. Coupled QM/MM molecular dynamics simulations of HCl interacting with ice surfaces and water clusters—Evidence of rapid ionization. J. Phys. Chem. A 104, 5787–5798 (2000)
Mantz, Y. A. et al. The interaction of HCl with the (0001) face of hexagonal ice studied theoretically via Car-Parrinello molecular dynamics. Chem. Phys. Lett. 348, 285–292 (2001)
Devlin, J. P. & Buch, V. Surface of ice as viewed from combined spectroscopic and computer modeling studies. J. Phys. Chem. 99, 16534–16548 (1995)
Zundel, G. Hydrogen bonds with large proton polarizability and proton transfer processes in electrochemistry and biology. Adv. Chem. Phys. 111, 1–217 (2000)
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983)
Votava, C., Ahlrichs, R. & Geiger, A. The HCl-HCl interaction: From quantum mechanical calculations to properties of the liquid. J. Chem. Phys. 78, 6841–6848 (1983)
Uras, N., Buch, V. & Devlin, J. P. Hydrogen bond surface chemistry: interaction of NH3 with an ice particle. J. Phys. Chem. B 104, 9203–9209 (2000)
Devlin, J. P., Joyce, C. & Buch, V. Infrared spectra and structures of large water clusters. J. Phys. Chem. A 104, 1974–1977 (2000)
Agmon, N. The Grotthus mechanism. Chem. Phys. Lett. 244, 456–462 (1995)
Ando, K. & Hynes, J. T. Molecular mechanism of HCl acid ionization in water. J. Phys. Chem. B 101, 10464–10478 (1997)
Lundgren, J. O. & Olovsson, I. The crystal structure of hydrogen chloride dihydrate. Acta Crystallogr. 23, 966–971 (1967)
Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. J. Chem. Phys. 90, 1007–1023 (1989)
Frisch, M. J. et al. Gaussian 98 (Gaussian, Pittsburgh, 1998)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Devlin, J., Uras, N., Sadlej, J. et al. Discrete stages in the solvation and ionization of hydrogen chloride adsorbed on ice particles. Nature 417, 269–271 (2002). https://doi.org/10.1038/417269a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/417269a
This article is cited by
-
Hydrogen-bonding behavior of various conformations of the HNO3…(CH3OH)2 ternary system
Journal of Molecular Modeling (2018)
-
Quantum chemical study of atmospheric aggregates: HCl•HNO3•H2SO4
Journal of Molecular Modeling (2014)
-
Large variation of vacancy formation energies in the surface of crystalline ice
Nature Materials (2011)
-
Dissociation, solvation, and dynamics of HBr in small water clusters
Theoretical Chemistry Accounts (2005)
-
Acids caught in the act
Nature (2002)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.