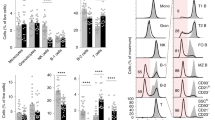
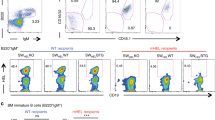
Abstract
Interaction of a B cell expressing self-specific B-cell antigen receptor (BCR) with an auto-antigen results in either clonal deletion or functional inactivation1,2,3. Both of these processes lead to B-cell tolerance and are essential for the prevention of auto-immune diseases. Whereas clonal deletion results in the death of developing autoreactive B cells, functional inactivation of self-reactive B lymphocytes leads to complex changes in the phenotype of peripheral B cells, described collectively as anergy1,2,3. Here we demonstrate that deficiency in protein kinase Cδ (PKC-δ) prevents B-cell tolerance, and allows maturation and terminal differentiation of self-reactive B cells in the presence of the tolerizing antigen. The importance of PKC-δ in B-cell tolerance is further underscored by the appearance of autoreactive anti-DNA and anti-nuclear antibodies in the serum of PKC-δ-deficient mice. As deficiency of PKC-δ does not affect BCR-mediated B-cell activation in vitro and in vivo, our data suggest a selective and essential role of PKC-δ in tolerogenic, but not immunogenic, B-cell responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Goodnow, C. C. et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature 334, 676–682 (1988).
Nemazee, D. A. & Burki, K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337, 562–566 (1989).
Hartley, S. B. et al. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353, 765–769 (1991).
Nishizuka, Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258, 607–614 (1992).
Le Good, J. A. et al. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281, 2042–2045 (1998).
Watanabe, T. et al. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-δ subspecies. Proc. Natl Acad. Sci. USA 89, 10159–10163 (1992).
Mischak, H. et al. Overexpression of protein kinase C-δ and -ε in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J. Biol. Chem. 268, 6090–6096 (1993).
Emoto, Y. et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 14, 6148–6156 (1995).
Ghayur, T. et al. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 184, 2399–2404 (1996).
Leitges, M. et al. Exacerbated vein graft arteriosclerosis in protein kinase Cδ-null mice. J. Clin. Invest. 108, 1505–1512 (2001).
Hippen, K. L., Tze, L. E. & Behrens, T. W. CD5 maintains tolerance in anergic B cells. J. Exp. Med. 191, 883–890 (2000).
Prodeus, A. P. et al. A critical role for complement in maintenance of self-tolerance. Immunity 9, 721–731 (1998).
Fulcher, D. A. et al. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J. Exp. Med. 183, 2313–2328 (1996).
Healy, J. I. et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity 6, 419–428 (1997).
Leitges, M. et al. Immunodeficiency in protein kinase C β-deficient mice. Science 273, 788–791 (1996).
Kang, S. W. et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 20, 5692–5702 (2001).
Doerre, S. & Corley, R. B. Constitutive nuclear translocation of NF-κB in B cells in the absence of IκB degradation. J. Immunol. 163, 269–277 (1999).
Glynne, R. et al. How self-tolerance and the immunosuppressive drug FK506 prevent B-cell mitogenesis. Nature 403, 672–676 (2000).
Chan, V. W. et al. The molecular mechanism of B cell activation by toll-like receptor protein RP-105. J. Exp. Med. 188, 93–101 (1998).
Nolan, G. P., Fiering, S., Nicolas, J. F. & Herzenberg, L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on β-d-galactosidase activity after transduction of Escherichia coli lacZ. Proc. Natl Acad. Sci. USA 85, 2603–2607 (1988).
Metzger, D. W., Ch’ng, L. K., Miller, A. & Sercarz, E. E. The expressed lysozyme-specific B cell repertoire. I. Heterogeneity in the monoclonal anti-hen egg white lysozyme specificity repertoire, and its difference from the in situ repertoire. Eur. J. Immunol. 14, 87–93 (1984).
Smith-Gill, S. J. et al. Mapping the antigenic epitope for a monoclonal antibody against lysozyme. J. Immunol. 128, 314–322 (1982).
Miyake, K., Yamashita, Y., Hitoshi, Y., Takatsu, K. & Kimoto, M. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J. Exp. Med. 180, 1217–1224 (1994).
Yamashita, Y. et al. A monoclonal antibody against a murine CD38 homologue delivers a signal to B cells for prolongation of survival and protection against apoptosis in vitro: unresponsiveness of X-linked immunodeficient B cells. Immunology 85, 248–255 (1995).
Lipford, G. B. et al. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J. Immunol. 27, 2340–2344 (1997).
Kozono, Y. et al. Cross-linking CD21/CD35 or CD19 increases both B7-1 and B7-2 expression on murine splenic B cells. J. Immunol. 160, 1565–1572 (1998).
Lyons, A. B. & Parish, C. R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171, 131–137 (1994).
Acknowledgements
We thank C. Goodnow, J. Cyster and F. Finkelman for providing reagents; members of the Laboratory for Lymphocyte Signaling in Cologne for technical assistance; and G. Hannon for the preparation of the manuscript. We thank M. Nussenzweig, D. O′Carrol, K. Rajewsky and C. Schmedt for discussion. This work was supported by The Irene Diamond Fund (A.T.) and National Institutes of Health grant (N-Y.Z. and A.T.), S. L. E. Foundation (K.S.), Graduiertenkolleg from the Deutsche Forschungsgemeinschaft, and The Rockefeller University's Women & Science Fellowship Program (I.M.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Supplementary information
Rights and permissions
About this article
Cite this article
Mecklenbräuker, I., Saijo, K., Zheng, NY. et al. Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature 416, 860–865 (2002). https://doi.org/10.1038/416860a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416860a
This article is cited by
-
Genetic association of PRKCD and CARD9 polymorphisms with Vogt–Koyanagi–Harada disease in the Chinese Han population
Human Genomics (2023)
-
PKCδ regulates the vascular biology in diabetic atherosclerosis
Cell Communication and Signaling (2023)
-
Phenotypic Variability in PRKCD: a Review of the Literature
Journal of Clinical Immunology (2023)
-
Equivocal, explicit and emergent actions of PKC isoforms in cancer
Nature Reviews Cancer (2021)
-
Systemic Lupus Erythematosus in Children and Young People
Current Rheumatology Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.