Abstract
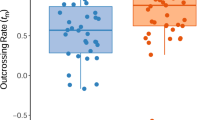
The transition from outcrossing to self-fertilization is one of the most common evolutionary trends in plants1. Reproductive assurance, where self-fertilization ensures seed production when pollinators and/or potential mates are scarce, is the most long-standing and most widely accepted explanation for the evolution of selfing2,3,4,5,6,7,8, but there have been few experimental tests of this hypothesis. Moreover, many apparently adaptive floral mechanisms that ensure the autonomous production of selfed seed might use ovules that would have otherwise been outcrossed. This seed discounting is costly if selfed offspring are less viable than their outcrossed counterparts, as often happens. The fertility benefit of reproductive assurance has never been examined in the light of seed discounting. Here we combine experimental measures of reproductive assurance with marker-gene estimates of self-fertilization, seed discounting and inbreeding depression to show that, during 2 years in 10 Ontario populations of Aquilegia canadensis (Ranunculaceae), reproductive assurance through self-fertilization increases seed production, but this benefit is greatly outweighed by severe seed discounting and inbreeding depression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Stebbins, G. L. Flowering Plants: Evolution above the Species Level (Belknap, Cambridge, Massachusetts, 1974).
Darwin, C. R. The Effects of Cross and Self-fertilization in the Vegetable Kingdom (John Murray, London, 1876).
Baker, H. G. Self-compatibility and establishment after ‘long-distance’ dispersal. Evolution 9, 347–348 (1955).
Jain, S. K. The evolution of inbreeding in plants. Annu. Rev. Ecol. Syst. 7, 69–95 (1976).
Lloyd, D. G. in Demography and Evolution in Plant Populations (ed. Solbrig, O. T.) 67–88 (Blackwell, Oxford, 1980).
Yahara, T. Graphical analysis of mating system evolution in plants. Evolution 46, 557–561 (1992).
Jarne, P. & Charlesworth, D. The evolution of the selfing rate in functionally hermaphroditic plants and animals. Annu. Rev. Ecol. Syst. 24, 441–466 (1993).
Holsinger, K. E. Pollination biology and the evolution of mating systems in flowering plants. Evol. Biol. 29, 107–149 (1996).
Fisher, R. A. Average excess and average effect of a gene substitution. Ann. Eugen. 11, 53–63 (1941).
Charlesworth, D. & Charlesworth, B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268 (1987).
Barrett, S. C. H., Harder, L. D. & Worley, A. C. The comparative biology of pollination and mating in flowering plants. Phil. Trans. R. Soc. Lond. B 351, 1271–1280 (1996).
Lande, R., Schemske, D. W. & Schultz, S. T. High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations. Evolution 48, 965–978 (1994).
Husband, B. C. & Schemske, D. W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50, 54–70 (1996).
Lloyd, D. G. Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int. J. Plant Sci. 153, 370–380 (1992).
Schoen, D. J., Morgan, M. T. & Bataillon, T. How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Phil. Trans. R. Soc. Lond. B 351, 1281–1290 (1996).
Schoen, D. J. & Brown, A. H. D. Whole- and part-flower self-pollination in Glycine clandestina and G. argyrea and the evolution of autogamy. Evolution 45, 1665–1674 (1991).
Eckert, C. G. & Schaefer, A. Does self-pollination provide reproductive assurance in wild columbine, Aquilegia canadensis (Ranunculaceae)? Am. J. Bot. 85, 919–924 (1998).
Cruden, R. W. & Lyon, D. L. in The Evolutionary Ecology of Plants (eds Bock, J. H. & Linhart, Y. B.) 171–208 (Westview Press, Boulder, Colorado, 1989).
Piper, J. G., Charlesworth, B. & Charlesworth, D. A high rate of self-fertilization and increased seed fertility of homostyle primroses. Nature 310, 50–51 (1984).
Piper, J. G., Charlesworth, B. & Charlesworth, D. Breeding system evolution in Primula vulgaris and the role of reproductive assurance. Heredity 56, 207–217 (1986).
Routley, M. B., Mavraganis, K. & Eckert, C. G. Effect of population size on the mating system in a self-compatible, autogamous plant, Aquilegia canadensis (Ranunculaceae). Heredity 82, 518–528 (1999).
Mavraganis, K. & Eckert, C. G. Effect of population size and isolation on reproductive output of Aquilegia canadensis (Ranunculaceae). Oikos 95, 533–546 (2001).
Macior, L. W. The pollination of vernal angiosperms. Oikos 30, 452–460 (1978).
Lloyd, D. G. & Schoen, D. J. Self- and cross-fertilization in plants. I. Functional dimensions. Int. J. Plant Sci. 153, 358–369 (1992).
Griffin, S. R., Mavraganis, K. & Eckert, C. G. Experimental analysis of protogyny in Aquilegia canadensis. Am. J. Bot. 87, 1246–1256 (2000).
Morgan, M. T., Schoen, D. J. & Bataillon, T. M. The evolution of self-fertilization in perennials. Am. Nat. 150, 618–638 (1997).
Morgan, M. T., and Schoen, D. J. The role of theory in an emerging new plant reproductive biology. Trends Ecol. Evol. 12, 231–234 (1997).
Ritland, K. A series of FORTRAN computer programs for estimating plant mating systems. J. Hered. 81, 235–237 (1990).
Schoen, D. J. & Lloyd, D. G. Self- and cross-fertilization in plants. III. Methods for studying modes and functional aspects of self-fertilization. Int. J. Plant Sci. 153, 381–393 (1992).
Ritland, K. Inferences about inbreeding depression based on changes of the inbreeding coefficient. Evolution 44, 1230–1241 (1990).
Acknowledgements
We thank M. Bhardwaj, S. Griffin and A. Kliber for help with the field work; C. Muis and M. Bhardwaj for help in the laboratory; and S. Barrett, K. Holsinger, A. Kliber, B. Montgomerie and M. Morgan for comments on the manuscript. This work was supported in the field by the Queen's University Biological Station and by a research grant from the Natural Sciences and Engineering Research Council of Canada to C.G.E.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herlihy, C., Eckert, C. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416, 320–323 (2002). https://doi.org/10.1038/416320a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/416320a
This article is cited by
-
Population size as a major determinant of mating system and population genetic differentiation in a narrow endemic chasmophyte
BMC Plant Biology (2023)
-
Genomic evidence reveals high genetic diversity in a narrowly distributed species and natural hybridization risk with a widespread species in the genus Geodorum
BMC Plant Biology (2023)
-
Compatibility systems and pollinator dependency in morning glory species (Convolvulaceae)
BMC Plant Biology (2023)
-
Evidence of local adaptation despite strong drift in a Neotropical patchily distributed bromeliad
Heredity (2021)
-
Genetic structure and diversity of the mustard hill coral Porites astreoides along the Florida Keys reef tract
Marine Biodiversity (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.