Abstract
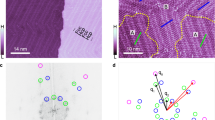
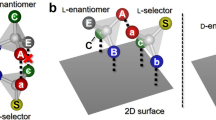
Stereochemistry plays a central role in controlling molecular recognition and interaction: the chemical and biological properties of molecules depend not only on the nature of their constituent atoms but also on how these atoms are positioned in space. Chiral specificity is consequently fundamental in chemical biology and pharmacology1,2 and has accordingly been widely studied. Advances in scanning probe microscopies now make it possible to probe chiral phenomena at surfaces at the molecular level. These methods have been used to determine the chirality of adsorbed molecules3,4,5, and to provide direct evidence for chiral discrimination in molecular interactions6 and the spontaneous resolution of adsorbates into extended enantiomerically pure overlayers3,7,8,9. Here we report scanning tunnelling microscopy studies of cysteine adsorbed to a (110) gold surface, which show that molecular pairs formed from a racemic mixture of this naturally occurring amino acid are exclusively homochiral, and that their binding to the gold surface is associated with local surface restructuring. Density-functional theory10 calculations indicate that the chiral specificity of the dimer formation process is driven by the optimization of three bonds on each cysteine molecule. These findings thus provide a clear molecular-level illustration of the well known three-point contact model11,12 for chiral recognition in a simple bimolecular system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sheldon, R. A. Chirotechnology 39–72 (Dekker, New York/Basel, 1993).
Cline, D. B. Physical Origin of Homochirality in Life 17–49 (AIP Press, Woodbury, New York, 1996).
Fang, H., Giancarlo, L. C. & Flynn, G. W. Direct determination of the chirality of organic molecules by scanning tunneling microscopy. J. Phys. Chem. 102, 7311–7315 (1998).
Lopinski, G. P., Moffatt, D. J., Wayner, D. D. M. & Wolkow, R. A. Determination of the absolute chirality of individual adsorbed molecules using the scanning tunneling microscope. Nature 392, 909–911 (1998).
Böhringer, M., Morgenstern, K., Schneider, W.-D. & Berndt, R. Separation of a racemic mixture of two-dimensional molecular clusters by scanning tuneling microscopy. Angew. Chem. Int. Edn 38, 821–823 (1999).
McKendry, R., Theoclitou, M.-E., Rayment, T. & Abell, Ch. Chiral discrimination by chemical force microscopy. Nature 391, 566–569 (1998).
Lorenzo, M. O., Baddeley, C. J., Muryn, C. & Raval, R. Extended surface chirality from supramolecular assemblies of adsorbed chiral molecules. Nature 404, 376–379 (2000).
Chen, Q., Lee, C. W., Frankel, D. J. & Richardson, N. V. The formation of enantiospecific phases on a Cu{110} surface. PhysChemComm [online] 9, (1999).
Eckhardt, C. J. et al. Separation of chiral phases in monolayer crystals of racemic amphiphiles. Nature 362, 614–616 (1993).
Payne, M. C., Teter, M. P., Allan, D. C., Arias, T. A. & Joannopoulos, J. D. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients. Rev. Mod. Phys. 64, 1045–1097 (1992).
Easson, L. H. & Stedman, E. Studies on the relationship between chemical constitution and physiological action. Biochem. 27, 1257–1266 (1933).
Booth, T. D., Wahnon, D. & Wainer, I. W. Is chiral recognition a three-point process? Chirality 9, 96–98 (1997).
Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 96, 1533–1554 (1996).
Poirier, G. E. & Pylant, E. D. The self-assembly mechanism of alkanethiols on Au(111). Science 272, 1145–1148 (1996).
Gritsch, T., Coulman, D., Behm, J. R. & Ertl, G. A. A scanning tunneling microscopy investigation of the structure of the Pt(110) and Au(110) surfaces. Surf. Sci. 257, 297–306 (1991).
Grönbeck, H., Curioni, A. & Andreoni, W. Thiols and disulfides on the Au(111) surface: the headgroup-gold interaction. J. Am. Chem. Soc. 122, 3839–3842 (2000).
Tersoff, J. & Hamann, D. R. Theory of the scanning tunneling microscopy. Phys. Rev. B 31, 805–813 (1985).
Gottschalck, J. & Hammer, B. A density functional theory study of the adsorption of sulfur, mercapto and methylthiolate on Au(111). J. Chem. Phys. 116, 784–790 (2002).
Lægsgaard, E., Besenbacher, F., Mortensen, K. & Stensgaard, I. A fully automated, ‘thimble-size’ scanning tunnelling microscope. J. Microscopy 152, 663–669 (1988).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Acknowledgements
This work was supported by the Danish National Research Foundation through the Center for Atomic-scale Materials Physics (CAMP) and by the Danish Natural Science Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kühnle, A., Linderoth, T., Hammer, B. et al. Chiral recognition in dimerization of adsorbed cysteine observed by scanning tunnelling microscopy. Nature 415, 891–893 (2002). https://doi.org/10.1038/415891a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415891a
This article is cited by
-
Kinetic control over the chiral-selectivity in the formation of organometallic polymers on a Ag(110) surface
Communications Chemistry (2024)
-
Chirality control of a single carbene molecule by tip-induced van der Waals interactions
Nature Communications (2023)
-
Chiral metal nanostructures: synthesis, properties and applications
Rare Metals (2023)
-
Evolution of Br⋯Br contacts in enantioselective molecular recognition during chiral 2D crystallization
Nature Communications (2022)
-
Simultaneous switching of supramolecular chirality and organizational chirality driven by Coulomb expansion
Nano Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.