Abstract
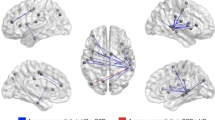
Brief intensive cognitive-behavioral therapy (CBT) using exposure and response prevention significantly improves obsessive-compulsive disorder (OCD) symptoms in as little as 4 weeks. However, it has been thought that much longer treatment was needed to produce the changes in brain function seen in neuroimaging studies of OCD. We sought to elucidate the brain mediation of response to brief intensive CBT for OCD and determine whether this treatment could induce functional brain changes previously seen after longer trials of pharmacotherapy or standard CBT. [18F]-fluorodeoxyglucose positron emission tomography brain scans were obtained on 10 OCD patients before and after 4 weeks of intensive individual CBT. Twelve normal controls were scanned twice, several weeks apart, without treatment. Regional glucose metabolic changes were compared between groups. OCD symptoms, depression, anxiety and overall functioning improved robustly with treatment. Significant changes in normalized regional glucose metabolism were seen after brief intensive CBT (P=0.04). Compared to controls, OCD patients showed significant bilateral decreases in normalized thalamic metabolism with intensive CBT but had a significant increase in right dorsal anterior cingulate cortex activity that correlated strongly with the degree of improvement in OCD symptoms (P=0.02). The rapid response of OCD to intensive CBT is mediated by a distinct pattern of changes in regional brain function. Reduction of thalamic activity may be a final common pathway for improvement in OCD, but response to intensive CBT may require activation of dorsal anterior cingulate cortex, a region involved in reappraisal and suppression of negative emotions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Baxter LR, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE . Local cerebral glucose metabolic rates in obsessive-compulsive disorder—a comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry 1987; 44: 211–218.
Nordahl TE, Benkelfat C, Semple WE, Gross M, King AC, Cohen RM . Cerebral glucose metabolic rates in obsessive-compulsive disorder. Neuropsychopharmacology 1989; 2: 23–28.
Swedo S, Schapiro MG, Grady CL, Cheslow DL, Leonard HL, Kumar A et al. Cerebral glucose metabolism in childhood onset obsessive-compulsive disorder. Arch Gen Psychiatry 1989; 46: 518–523.
Sawle GV, Hymas NF, Lees AJ, Frackowiak RS . Obsessional slowness: functional studies with positron emission tomography. Brain 1991; 114: 2191–2202.
Perani D, Colombo C, Bressi S, Bonfanti A, Grassi F, Scarone S et al. [18F]FDG PET study in obsessive-compulsive disorder: a clinical/metabolic correlation study after treatment. Br J Psychiatry 1995; 166: 244–250.
Saxena S, Brody AL, Ho ML, Alborzian S, Ho MK, Maidment KM et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently. Biol Psychiatry 2001; 50: 159–170.
Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS et al. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res: Neuroimaging 2003; 122: 37–47.
Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM . Local cerebral glucose metabolic rates in obsessive-compulsive disorder: patients treated with clomipramine. Arch Gen Psychiatry 1990; 47: 840–848.
Baxter LR, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziota JC et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49: 681–689.
Swedo SE, Pietrini P, Leonard HL, Schapiro MB, Rettew DC, Goldberger EL et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry 1992; 49: 690–694.
Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder versus major depression. Arch Gen Psychiatry 2002; 59: 250–261.
Hansen ES, Hasselbach S, Law I, Bolwig TG . The caudate nucleus in obsessive-compulsive disorder. Reduced metabolism following treatment with paroxetine: a PET study. Int J Neuropsychopharmacol 2001; 5: 1–10.
Kang DH, Kwon JS, Kim JJ, Youn T, Park HJ, Kim MS et al. Brain glucose metabolic changes associated with neuropsychological improvements after 4 months of treatment in patients with obsessive-compulsive disorder. Acta Psychiatr Scand 2003; 107: 291–297.
Diler RS, Kibar M, Ayse A . Pharmacotherapy and regional cerebral blood flow in children with obsessive-compulsive disorder. Yonsei Med J 2004; 45: 90–99.
Ho Pian KL, van Megan HJGM, Ramsy NF, Mandl R, van Rijk PP, Wynee HJ et al. Decreased thalamic blood flow in obsessive-compulsive disorder patients responding to fluvoxamine. Psychiatry Res: Neuroimaging 2005; 138: 89–97.
Schwartz JM, Stoessel PW, Baxter LR, Martin KM, Phelps ME . Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53: 109–113.
Nakatani E, Nakgawa A, Ohara Y, Goto S, Uozumi N, Iwakiri M et al. Effects of behavior therapy on regional cerebral blood flow in obsessive-compulsive disorder. Psychiatry Res: Neuroimaging 2003; 124: 113–120.
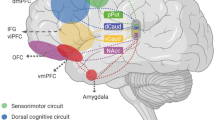
Alexander GE, DeLong MR, Strick PL . Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 1986; 9: 357–381.
Saxena S, Brody AL, Schwartz JM, Baxter LR . Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry 1998; 173 (Suppl 35): 26–38.
Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC . Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 1995; 52: 53–60.
el Mansari M, Bouchard C, Blier P . Alteration of serotonin release in the guinea pig orbito-frontal cortex by selective serotonin reuptake inhibitors. Relevance to treatment of obsessive-compulsive disorder. Neuropsychopharmacology 1995; 13: 117–127.
Marks I . Behaviour therapy for obsessive-compulsive disorder: a decade of progress. Can J Psychiatry 1997; 42: 1021–1027.
Foa EB, Goldstein A . Continuous exposure and complete response prevention in the treatment of obsessive-compulsive neurosis. Behav Ther 1978; 9: 821–829.
Lindsay M, Crino R, Andrews G . Controlled trial of exposure and response prevention in obsessive-compulsive disorder. Br J Psychiatry 1996; 171: 135–139.
Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME et al. Randomized, placebo-controlled trial of exposure and ritual prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry 2005; 162: 151–161.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P). Biometrics Research Department, New York State Psychiatric Institute, New York, 1996.
Goodman WK, Price LH, Rasmussen SA, Mazure C . The Yale-Brown Obsessive Compulsive Scale I: development, use, and reliability. Arch Gen Psychiatry 1989; 46: 1006–1011.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Hamilton M . The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–55.
Endicott J, Spitzer RL, Fleiss JL, Cohen J . The Global Assessment Scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 1976; 41: 586–601.
Guy W . The clinical global impressions scale. In: United States Department of Health, Education, and Welfare. ECDEU Assessment Manual for Psychopharmacology. National Institute of Mental Health: Rockville, MD, 1976, pp. 217–222.
Pallanti S, Hollander E, Bienstock C, Koran L, Leckman J et al. International treatment refractory OCD consortium. Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol 2002; 5: 181–191.
Damasio H . Human Brain Anatomy in Computerized Images. Oxford University Press: New York, 1995.
Mai JK, Assheuer J, Paxton DA . Atlas of the Human Brain. Academic Press, Harcourt Brace and Company: San Diego, CA, 1997.
Rajkowska G, Goldman-Rakic PS . Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Tailarach Coordinate System. Cereb Cortex 1995; 5: 323–337.
Biver F, Goldman S, Francois A, De La Porte C, Luxen A, Gribomont B et al. Changes in metabolism of cerebral glucose after stereotactic leukotomy for refractory obsessive-compulsive disorder: a case report. J Neurol Neurosurg Psychiatry 1995; 58: 502–505.
Sachdev P, Trollor J, Walker A, Wen W, Fulham M, Smith JS et al. Bilateral orbitomedial leucotomy for obsessive-compulsive disorder: a single-case study using positron emission tomography. Aust N Z J Psychiatry 2001; 35: 684–690.
Saxena S, Bota RG, Brody AL . Brain–behavior relationships in obsessive-compulsive disorder. Sem Clin Neuropsychiatry 2001; 6: 82–101.
Saxena S, O’Neill J, Rauch SL . The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt BA (ed). Cingulate Neurobiology and Disease, vol. 1, Oxford University Press: Oxford, New York, NY, 2007, in press.
Hoehn-Saric R, Pearlson GD, Harris GJ, Machlin SR, Camargo EE . Effects of fluoxetine on regional cerebral blood flow in obsessive-compulsive patients. Am J Psychiatry 1991; 148: 1243–1245.
Hoehn-Saric R, Schlaepfer TE, Greenberg BD, McLeod DR, Pearlson GD, Wong SH . Cerebral blood flow in obsessive compulsive patients with major depression: effect of treatment with sertraline or desipramine on treatment responders and non-responders. Psychiatry Res: Neuroimaging 2001; 108: 89–100.
Mindus P, Ericson K, Greitz T, Meyerson BA, Nyman H, Sjogren I . Regional cerebral glucose metabolism in anxiety disorders studied with positron emission tomography before and after psychosurgical intervention. A preliminary report. Acta Radiol Suppl 1986; 369: 444–448.
Rubin RT, Villanueva-Meyer J, Ananth J, Trajmar PG, Mena I . Regional 133Xe cerebral blood flow and cerebral 99m-HMPAO uptake in unmedicated obsessive-compulsive disorder patients and matched normal control subjects: determination by high-resolution single-photon emission computed tomography. Arch Gen Psychiatry 1992; 49: 695–702.
Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 57: 901–910.
Viard A, Flament MF, Artiges E, Dehaene S, Naccache L, Cohen D et al. Cognitive control in childhood-onset obsessive-compulsive disorder: a functional MRI study. Psychol Med 2005; 35: 1007–1017.
Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S et al. Modulation of cortical-limbic pathways in major depression. Arch Gen Psychiatry 2004; 61: 34–41.
Vogt BA, Vogt L, Farber NB, Bush G . Architecture and neurocytology of monkey cingulate cortex. Neurology 2005; 485: 218–239.
Vogt BA, Finch DM, Olson CR . Functional heterogeneity in the cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2: 435–443.
Vogt BA, Berger GR, Derbyshire SWJ . Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci 2003; 18: 3134–3144.
Lane RD, Fink GR, Chau PM-L, Dolan RJ . Neural activation during selective attention to subjective emotional responses. Neuroreport 1997; 8: 3969–3972.
Gusnard DA, Akbudak E, Shulman GL, Raichle ME . Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98: 4259–4264.
Ochsner K, Bunge SA, Gross JJ, Gabrieli JDE . Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cog Neurosci 2002; 14: 1215–1229.
Beauregard M, Levesque J, Bourgouin P . Neural correlates of conscious self-regulation of emotion. J Neurosci 2001; 21: RC165.
Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME . Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry 2005; 57: 210–219.
Paus T . Primate anterior cingulate cortex: where motor control, drive, and cognition interface. Nature Rev: Neurosci 2001; 2: 417–424.
Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR . Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 2003; 53: 494–501.
Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53: 595–606.
Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM . fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 2000; 34: 317–324.
van den Heuvel OA, Veltman DJ, Groenewegen HJ, Dolan RJ, Cath DC, Boellaard R et al. Amygdala activity in obsessive-compulsive disorder with contamination fear: a study with oxygen-15 water positron emission tomography. Psychiatry Res 2004; 132: 225–237.
Awh E, Gehring WJ . The anterior cingulate lends a hand in response selection. Nature Neurosci 1999; 2: 853–854.
Carter CS, Braver TS, Barch DM, Botvinik MM, Noll D, Cohen J . Anterior cingulate cortex, error detection, and online monitoring of performance. Science 1998; 280: 747–749.
Devinsky O, Morrell MJ, Vogt BA . Contributions of anterior cingulate cortex to behavior. Brain 1995; 118: 279–306.
Baxter LR, Ackermann RF, Swerdlow NR, Brody AL, Saxena S, Schwartz JM et al. Specific brain system mediation of OCD responsive to either medication or behavior therapy. In: Goodman W, Rudorfer M, Maser J (eds). Obsessive-Compulsive Disorder: Contemporary Issues in Treatment. Lawrence Erlbaum Associates Inc.: Mahwah, NJ, 2000, pp. 573–609.
Pigott TA, Seay SM . A review of the efficacy of selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Clin Psychiatry 1999; 60: 101–106.
Warach S, Gur RC, Gur RE, Skolnick BE, Obrist WD, Reivich M . The reproducibility of the 133Xe inhalation technique in resting studies: task order and sex related effects in healthy young adults. J Cereb Blood Flow Metab 1987; 7: 702–708.
Metz JT, Odeh N, Cooper MD . ‘First session’ effects in PET studies. J Nucl Med 1989; 30: 899.
Warach S, Gur RC, Gur RE, Skolnick BE, Obrist WD, Reivich M . Decreases in frontal and parietal lobe regional cerebral blood flow related to habituation. J Cereb Blood Flow Metab 1992; 12: 546–553.
Stapleton JM, Morgan MJ, Liu X, Yung BC, Phillips RL, Wong DF et al. Cerebral glucose utilization is reduced in second test session. J Cereb Blood Flow Metab 1997; 17: 704–712.
Mosconi L, Tsui W-H, De Santi S, Li J, Rusinek H, Convit A et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 2005; 64: 1860–1867.
Sun FT, Schriber RA, Greenia JM, He J, Gitcho A, Jagust WJ . Automated template-based PET region of interest analyses in the aging brain. NeuroImage 2007; 34: 608–617.
Acknowledgements
This work was supported by an NIMH grant (R01 MH069433) to Dr Saxena and by donations to the Westwood Institute for Anxiety Disorders (Dr Gorbis). For generous support, we thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M and Linda R Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund and the National Center for Research Resources grants RR12169, RR13642 and RR08655. This work was presented in part at the American College of Neuropsychopharmacology 43rd Annual Meeting (San Juan, Puerto Rico, 15 December 2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saxena, S., Gorbis, E., O'Neill, J. et al. Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Mol Psychiatry 14, 197–205 (2009). https://doi.org/10.1038/sj.mp.4002134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4002134
Keywords
This article is cited by
-
Gamma knife capsulotomy for intractable OCD: Neuroimage analysis of lesion size, location, and clinical response
Translational Psychiatry (2023)
-
Decreased left amygdala functional connectivity by cognitive-coping therapy in obsessive-compulsive disorder
Molecular Psychiatry (2021)
-
FMRI hemodynamic response function (HRF) as a novel marker of brain function: applications for understanding obsessive-compulsive disorder pathology and treatment response
Brain Imaging and Behavior (2021)
-
Glutamate in Pediatric Obsessive-Compulsive Disorder and Response to Cognitive-Behavioral Therapy: Randomized Clinical Trial
Neuropsychopharmacology (2017)
-
Mechanisms of cognitive-behavioral therapy for obsessive-compulsive disorder involve robust and extensive increases in brain network connectivity
Translational Psychiatry (2017)