Abstract
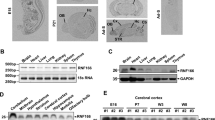
Disrupted-in-schizophrenia 1 (DISC1) is a gene disrupted by a (1;11) (q42.1;q14.3) translocation that segregates with major psychiatric disorders in a Scottish family. To investigate how DISC1 confers susceptibility to psychiatric disorders, we previously identified fasciculation and elongation protein zeta-1 and Kendrin as DISC1-interacting molecules in a yeast two-hybrid screen of a human brain complementary DNA library. Here, we have further identified a novel DISC1-interacting protein, termed DISC1-Binding Zinc-finger protein (DBZ), which has a predicted C2H2-type zinc-finger motif and coiled-coil domains. DBZ was co-immunoprecipitated with DISC1 in lysates of PC12 cells and rat brain tissue. The domain of DISC1 interacting with DBZ was close to the translocation breakpoint in the DISC1 gene. DBZ messenger RNA (mRNA) was expressed in human brains, but not in peripheral tissues. In situ hybridization revealed high expression of DBZ mRNA in the hippocampus, olfactory tubercle, cerebral cortex and striatum in rats. Because this pattern of localization was similar to that of the pituitary adenylate cyclase (PAC1) receptor for pituitary adenylate cyclase-activating polypeptide (PACAP), which has recently been implicated in neuropsychological functions, we examined whether DISC1/DBZ interaction was involved in the PACAP signaling pathway. PACAP upregulated DISC1 expression and markedly reduced the association between DISC1 and DBZ in PC12 cells. A DISC1-binding domain of DBZ reduced the neurite length in PC12 cells after PACAP stimulation and in primary cultured hippocampal neurons. The present results provide some new molecular insights into the mechanisms of neuronal development and neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Weinberger DR . Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44: 660–669.
Lewis DA, Lieberman JA . Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28: 325–334.
Frankle WG, Lerma J, Laruelle M . The synaptic hypothesis of schizophrenia. Neuron 2003; 39: 205–216.
Heinz A, Romero B, Gallinat J, Juckel G, Weinberger DR . Molecular brain imaging and the neurobiology and genetics of schizophrenia. Pharmacopsychiatry 2003; 36: S152–S157.
Mueser KT, McGurk SR . Schizophrenia. Lancet 2004; 363: 2063–2072.
Harrison PJ, Weinberger DR . Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 2005; 10: 40–68.
Millar JK, Wilson-Annan JC, Anderson S, Christie S, Taylor MS, Semple CA et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 2000; 9: 1415–1423.
Millar JK, Christie S, Anderson S, Lawson D, Hsiao-Wei-Loh D, Devon RS et al. Genomic structure and localisation within a linkage hotspot of disrupted in schizophrenia 1, a gene disrupted by a translocation segregating with schizophrenia. Mol Psychiatry 2001; 6: 173–178.
Sachs NA, Sawa A, Holmes SE, Ross CA, DeLisi LE, Margolis RL . A frameshift mutation in disrupted in schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry 2005; 10: 758–764.
Hodgkinson CA, Goldman D, Jaeger J, Persaud S, Kane JM, Lipsky RH et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet 2004; 75: 862–872.
Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T et al. Disrupted-in-schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry 2003; 8: 685–694.
Honda A, Miyoshi K, Baba K, Taniguchi M, Koyama Y, Kuroda S et al. Expression of fasciculation and elongation protein zeta-1 (FEZ1) in the developing rat brain. Brain Res Mol Brain Res 2004; 122: 89–92.
Miyoshi K, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Katayama T, Tohyama M et al. DISC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun 2004; 317: 1195–1199.
Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K . Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol 1999; 144: 403–411.
Yamada K, Nakamura K, Minabe Y, Iwayama-Shigeno Y, Takao H, Toyota T et al. Association analysis of FEZ1 variants with schizophrenia in Japanese cohorts. Biol Psychiatry 2004; 56: 683–690.
Arimura A . Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 1998; 48: 301–331.
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H . Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 2000; 52: 269–324.
Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc Natl Acad Sci USA 2001; 98: 13355–13360.
Tanaka K, Shintani N, Hashimoto H, Kawagishi N, Ago Y, Matsuda T et al. Psychostimulant-induced attenuation of hyperactivity and prepulse inhibition deficits in Adcyap1-deficient mice. J Neurosci 2006; 26: 5091–5097.
Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A . Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. NeuroReport 2003; 14: 2095–2098.
Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci 2001; 21: 5520–5527.
Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G . Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res 2001; 92: 78–84.
Nicot A, Otto T, Brabet P, Dicicco-Bloom EM . Altered social behavior in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. J Neurosci 2004; 24: 8786–8795.
Yamashita T, Shimada S, Guo W, Sato K, Kohmura E, Hayakawa T et al. Cloning and functional expression of a brain peptide/histidine transporter. J Biol Chem 1997; 272: 10205–10211.
Matsuo N, Ogawa S, Takagi T, Wanaka A, Mori T, Matsuyama T et al. Cloning of a putative vesicle transport-related protein, RA410, from cultured rat astrocytes and its expression in ischemic rat brain. J Biol Chem 1997; 272: 16438–16444.
Morris JA, Kandpal G, Ma L, Austin CP . DISC1 (disrupted-in-schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet 2003; 12: 1591–1608.
Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K et al. Disrupted-in-schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci USA 2003; 100: 289–294.
Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N et al. Prediction of the coding sequences of unidentified human genes. XII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res 1998; 5: 355–364.
Gianfrancesco F, Esposito T, Ombra MN, Forabosco P, Maninchedda G, Fattorini M et al. Identification of a novel gene and a common variant associated with uric acid nephrolithiasis in a Sardinian genetic isolate. Am J Hum Genet 2003; 72: 1479–1491.
Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K et al. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 1996; 371: 567–577.
Rose A, Meier I . Scaffolds, levers, rods and springs: diverse cellular functions of long coiled-coil proteins. Cell Mol Life Sci 2004; 61: 1996–2009.
Acknowledgements
We thank Ms Arakawa, Ms Moriya and Ms Ohashi for preparing our experiments. This research was partly supported by the 21st century COE program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and by a grant from Sankyo Foundation of Life Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hattori, T., Baba, K., Matsuzaki, S. et al. A novel DISC1-interacting partner DISC1-Binding Zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol Psychiatry 12, 398–407 (2007). https://doi.org/10.1038/sj.mp.4001945
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001945
Keywords
This article is cited by
-
Convergence of independent DISC1 mutations on impaired neurite growth via decreased UNC5D expression
Translational Psychiatry (2018)
-
Increased dysbindin-1B isoform expression in schizophrenia and its propensity in aggresome formation
Cell Discovery (2015)
-
Reductions in synaptic proteins and selective alteration of prepulse inhibition in male C57BL/6 mice after postnatal administration of a VIP receptor (VIPR2) agonist
Psychopharmacology (2015)
-
Molecular basis of major psychiatric diseases such as schizophrenia and depression
Anatomical Science International (2015)
-
Impact of DISC1 variation on neuroanatomical and neurocognitive phenotypes
Molecular Psychiatry (2011)