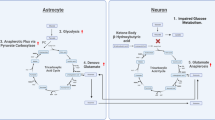
Abstract
Magnetic resonance spectroscopy (MRS) affords a noninvasive window on in vivo brain chemistry and, as such, provides a unique opportunity to gain insight into the biochemical pathology of bipolar disorder. Studies utilizing proton (1H) MRS have identified changes in cerebral concentrations of N-acetyl aspartate, glutamate/glutamine, choline-containing compounds, myo-inositol, and lactate in bipolar subjects compared to normal controls, while studies using phosphorus (31P) MRS have examined additional alterations in levels of phosphocreatine, phosphomonoesters, and intracellular pH. We hypothesize that the majority of MRS findings in bipolar subjects can be fit into a more cohesive bioenergetic and neurochemical model of bipolar illness that is both novel and yet in concordance with findings from complementary methodological approaches. In this review, we propose a hypothesis of mitochondrial dysfunction in bipolar disorder that involves impaired oxidative phosphorylation, a resultant shift toward glycolytic energy production, a decrease in total energy production and/or substrate availability, and altered phospholipid metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Belmaker RH . Bipolar disorder. N Engl J Med 2004; 351: 476–486.
Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA . Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biol Psychiatry 2000; 47: 475–481.
Birken DL, Oldendorf WH . N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 1989; 13: 23–31.
Urenjak J, Williams SR, Gadian DG, Noble M . Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci 1993; 13: 981–989.
Deicken RF, Pegues MP, Anzalone S, Feiwell R, Soher B . Lower concentration of hippocampal N-acetylaspartate in familial bipolar I disorder. Am J Psychiatry 2003; 160: 873–882.
Patel TB, Clark JB . Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 1979; 184: 539–546.
Truckenmiller ME, Namboodiri MA, Brownstein MJ, Neale JH . N-acetylation of L-aspartate in the nervous system: differential distribution of a specific enzyme. J Neurochem 1985; 45: 1658–1662.
Clark JB . N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 1998; 20: 271–276.
Baslow MH . N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res 2003; 28: 941–953.
Blakely RD, Coyle JT . The neurobiology of N-acetylaspartylglutamate. Int Rev Neurobiol 1988; 30: 39–100.
Madhavarao CN, Chinopoulos C, Chandrasekaran K, Namboodiri MA . Characterization of the N-acetylaspartate biosynthetic enzyme from rat brain. J Neurochem 2003; 86: 824–835.
van der Knaap MS, van der Grond J, Luyten PR, den Hollander JA, Nauta JJ, Valk J . 1H and 31P magnetic resonance spectroscopy of the brain in degenerative cerebral disorders. Ann Neurol 1992; 31: 202–211.
De Stefano N, Matthews PM, Arnold DL . Reversible decreases in N-acetylaspartate after acute brain injury. Magn Reson Med 1995; 34: 721–727.
Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R . N-acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma 2001; 18: 977–991.
Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A et al. N-acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem 2001; 77: 408–415.
Mathews PM, Andermann F, Silver K, Karpati G, Arnold DL . Proton MR spectroscopic characterization of differences in regional brain metabolic abnormalities in mitochondrial encephalomyopathies. Neurology 1993; 43: 2484–2490.
Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB . Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport 1996; 7: 1397–1400.
Brenner R, Bates TE, Davies SEC, Munro PMG, Williams SCR, Clark JB et al. Abnormal neuronal mitochondria: a cause of reduction in N-acetyl containing compounds (NA) in demyelinating disease. J Neurol 1994; 241: S29.
Cecil KM, DelBello MP, Morey R, Strakowski SM . Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord 2002; 4: 357–365.
Chang K, Adleman N, Dienes K, Barnea-Goraly N, Reiss A, Ketter T . Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry 2003; 53: 1059–1065.
Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J et al. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biol Psychiatry 2003; 53: 906–913.
O'Donnell T, Rotzinger S, Nakashima TT, Hanstock CC, Ulrich M, Silverstone PH . Chronic lithium and sodium valproate both decrease the concentration of myo-inositol and increase the concentration of inositol monophosphates in rat brain. Brain Res 2000; 880: 84–91.
Sharma R, Venkatasubramanian PN, Barany M, Davis JM . Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res 1992; 8: 43–49.
Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? Biol Psychiatry 2000; 48: 1–8.
Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC . Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int Clin Psychopharmacol 2003; 18: 73–79.
Brambilla P, Stanley JA, Sassi RB, Nicoletti MA, Mallinger AG, Keshavan MS et al. H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology 2004; 29: 1918–1924.
Friedman SD, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry 2004; 56: 340–348.
Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H et al. Proton magnetic resonance spectroscopy of the lenticular nuclei in bipolar I affective disorder. Psychiatry Res 1998; 84: 55–60.
Hamakawa H, Kato T, Shioiri T, Inubushi T, Kato N . Quantitative proton magnetic resonance spectroscopy of the bilateral frontal lobes in patients with bipolar disorder. Psychol Med 1999; 29: 639–644.
Castillo M, Kwock L, Courvoisie H, Hooper SR . Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. AJNR Am J Neuroradiol 2000; 21: 832–838.
Deicken RF, Eliaz Y, Feiwell R, Schuff N . Increased thalamic N-acetylaspartate in male patients with familial bipolar I disorder. Psychiatry Res 2001; 106: 35–45.
Zubieta JK, Huguelet P, Ohl F, Kilbourn MR, Koeppe RA, Frey KA . PET measures of monoaminergic synaptic density in bipolar I disorder: relationship with age of onset. Biol Psychiatry 1998; 43: S71.
Zubieta JK, Huguelet P, Ohl LE, Koeppe RA, Kilbourn MR, Carr JM et al. High vesicular monoamine transporter binding in asymptomatic bipolar I disorder: sex differences and cognitive correlates. Am J Psychiatry 2000; 157: 1619–1628.
Ongur D, Drevets WC, Price JL . Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 1998; 95: 13290–13295.
Sappey-Marinier D, Deicken RF, Fein G, Calabrese G, Hubesch B, Van Dyke C et al. Alterations in brain phosphorus metabolite concentrations associated with areas of high signal intensity in white matter at MR imaging. Radiology 1992; 183: 247–256.
Vion-Dury J, Meyerhoff DJ, Cozzone PJ, Weiner MW . What might be the impact on neurology of the analysis of brain metabolism by in vivo magnetic resonance spectroscopy? J Neurol 1994; 241: 354–371.
Brooke NS, Ouwerkerk R, Adams CB, Radda GK, Ledingham JG, Rajagopalan B . Phosphorus-31 magnetic resonance spectra reveal prolonged intracellular acidosis in the brain following subarachnoid hemorrhage. Proc Natl Acad Sci USA 1994; 91: 1903–1907.
Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K et al. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke 1995; 26: 1478–1489.
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995; 15: 961–973.
Fiskum G, Murphy AN, Beal MF . Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 1999; 19: 351–369.
Friberg H, Wieloch T . Mitochondrial permeability transition in acute neurodegeneration. Biochimie 2002; 84: 241–250.
Schinder AF, Olson EC, Spitzer NC, Montal M . Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 1996; 16: 6125–6133.
White RJ, Reynolds IJ . Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci 1996; 16: 5688–5697.
White RJ, Reynolds IJ . Mitochondria accumulate Ca2 + following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol 1997; 498(Part 1): 31–47.
Hamakawa H, Murashita J, Yamada N, Inubushi T, Kato N, Kato T . Reduced intracellular pH in the basal ganglia and whole brain measured by 31P-MRS in bipolar disorder. Psychiatry Clin Neurosci 2004; 58: 82–88.
Kato T, Takahashi S, Shioiri T, Inubushi T . Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo31P and 7Li magnetic resonance spectroscopy. J Affect Disord 1993; 27: 53–59.
Kato T, Takahashi S, Shioiri T, Inubushi T . Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord 1992; 26: 223–230.
Kato T, Murashita J, Kamiya A, Shioiri T, Kato N, Inubushi T . Decreased brain intracellular pH measured by 31P-MRS in bipolar disorder: a confirmation in drug-free patients and correlation with white matter hyperintensity. Eur Arch Psychiatry Clin Neurosci 1998; 248: 301–306.
Kato T, Takahashi S, Shioiri T, Murashita J, Hamakawa H, Inubushi T . Reduction of brain phosphocreatine in bipolar II disorder detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord 1994b; 31: 125–133.
Kato T, Shioiri T, Murashita J, Hamakawa H, Inubushi T, Takahashi S . Phosphorus-31 magnetic resonance spectroscopy and ventricular enlargement in bipolar disorder. Psychiatry Res 1994a; 55: 41–50.
Kato T, Inubushi T, Kato N . Prediction of lithium response by 31P-MRS in bipolar disorder. Int J Neuropsychopharmacol 2000; 3: 83–85.
Kato T, Kato N . Mitochondrial dysfunction in bipolar disorder. Bipolar Disord 2000; 2(3 Part 1): 180–190.
Smith GA, Brett CL, Church J . Effects of noradrenaline on intracellular pH in acutely dissociated adult rat hippocampal CA1 neurones. J Physiol 1998; 512(Part 2): 487–505.
Rudkin TM, Arnold DL . Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol 1999; 56: 919–926.
Lin DD, Crawford TO, Barker PB . Proton MR spectroscopy in the diagnostic evaluation of suspected mitochondrial disease. AJNR Am J Neuroradiol 2003; 24: 33–41.
Argov Z . Functional evaluation techniques in mitochondrial disorders. Eur Neurol 1998; 39: 65–71.
Barkovich AJ, Good WV, Koch TK, Berg BO . Mitochondrial disorders: analysis of their clinical and imaging characteristics. AJNR Am J Neuroradiol 1993; 14: 1119–1137.
Clausen T, Zauner A, Levasseur JE, Rice AC, Bullock R . Induced mitochondrial failure in the feline brain: implications for understanding acute post-traumatic metabolic events. Brain Res 2001; 908: 35–48.
Moore GJ, Galloway MP . Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull 2002; 36: 5–23.
Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry 2004; 61: 450–458.
Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W et al. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl) 2003; 168: 344–346.
Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, Shulman GI et al. Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1-13C]glucose. J Neurochem 1994; 63: 1377–1385.
Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR et al. Nonlinear decrease over time in N-acetyl aspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington's disease mice. J Neurochem 2000; 74: 2108–2119.
Cudkowicz ME, Martin JB, Koroshetz WJ . The neurology of Huntington's disease. In: Joseph AB, Young RR (eds). Movement Disorders in Neurology and Neuropsychiatry. Blackwell Science Inc.: Malden, MA, 1999, pp 147–154.
Ferrante RJ, Kowall NW, Beal MF, Martin JB, Bird ED, Richardson Jr EP . Morphologic and histochemical characteristics of a spared subset of striatal neurons in Huntington's disease. J Neuropathol Exp Neurol 1987; 46: 12–27.
Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB . Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 1986; 321: 168–171.
Taylor-Robinson SD, Weeks RA, Bryant DJ, Sargentoni J, Marcus CD, Harding AE et al. Proton magnetic resonance spectroscopy in Huntington's disease: evidence in favour of the glutamate excitotoxic theory. Mov Disord 1996; 11: 167–173.
de Graaf RA . Phosphorus-31 MRS. In: In vivo NMR Spectroscopy: Principles and Techniques. John Wiley & Sons Ltd: Chichester, England, 1998, pp 61–67.
Erecinska M, Silver IA . ATP and brain function. J Cereb Blood Flow Metab 1989; 9: 2–19.
Sauter A, Rudin M . Determination of creatine kinase kinetic parameters in rat brain by NMR magnetization transfer. Correlation with brain function. J Biol Chem 1993; 268: 13166–13171.
Kato T, Murashita J, Shioiri T, Hamakawa H, Inubushi T . Effect of photic stimulation on energy metabolism in the human brain measured by 31P-MR spectroscopy. J Neuropsychiatry Clin Neurosci 1996; 8: 417–422.
Rothman DL . 1H NMR studies of human brain metabolism and physiology. In: Gillies RJ (ed). NMR in Physiology and Biomedicine. Academic Press: San Diego, CA, 1994, pp 353–372.
Modica-Napolitano JS, Renshaw PF . Ethanolamine and phosphoethanolamine inhibit mitochondrial function in vitro: implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol Psychiatry 2004; 55: 273–277.
Eleff SM, Barker PB, Blackband SJ, Chatham JC, Lutz NW, Johns DR et al. Phosphorus magnetic resonance spectroscopy of patients with mitochondrial cytopathies demonstrates decreased levels of brain phosphocreatine. Ann Neurol 1990; 27: 626–630.
Barbiroli B, Montagna P, Martinelli P, Lodi R, Iotti S, Cortelli P et al. Defective brain energy metabolism shown by in vivo31P MR spectroscopy in 28 patients with mitochondrial cytopathies. J Cereb Blood Flow Metab 1993; 13: 469–474.
Barbiroli B, Montagna P, Cortelli P, Funicello R, Iotti S, Monari L et al. Abnormal brain and muscle energy metabolism shown by 31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 1992; 42: 1209–1214.
Welch KM, Barkley GL, Tepley N, Ramadan NM . Central neurogenic mechanisms of migraine. Neurology 1993; 43(6 Suppl 3): S21–S25.
Kato T, Shioiri T, Murashita J, Hamakawa H, Takahashi Y, Inubushi T et al. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol Med 1995; 25: 557–566.
Purdon AD, Rapoport SI . Energy requirements for two aspects of phospholipid metabolism in mammalian brain. Biochem J 1998; 335(Part 2): 313–318.
Govindaraju V, Young K, Maudsley AA . Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 2000; 13: 129–153.
Bluml S, Seymour KJ, Ross BD . Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med 1999; 42: 643–654.
Pouwels PJ, Frahm J . Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med 1998; 39: 53–60.
Tan J, Bluml S, Hoang T, Dubowitz D, Mevenkamp G, Ross B . Lack of effect of oral choline supplement on the concentrations of choline metabolites in human brain. Magn Reson Med 1998; 39: 1005–1010.
Wang Y, Li SJ . Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo1H magnetic resonance spectroscopy. Magn Reson Med 1998; 39: 28–33.
Freeman JJ, Jenden DJ . The source of choline for acetylcholine synthesis in brain. Life Sci 1976; 19: 949–961.
Miller BL . A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 1991; 4: 47–52.
Ackerstaff E, Glunde K, Bhujwalla ZM . Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem 2003; 90: 525–533.
Farber SA, Slack BE, Blusztajn JK . Acceleration of phosphatidylcholine synthesis and breakdown by inhibitors of mitochondrial function in neuronal cells: a model of the membrane defect of Alzheimer's disease. FASEB J 2000; 14: 2198–2206.
Stoll A, Cohen B, Hanin I . Erythrocyte choline concentration in psychiatric disorders. Biol Psychiatry 1991; 29: 309–321.
Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC, Silverstone PH . Brain choline concentrations may not be altered in euthymic bipolar disorder patients chronically treated with either lithium or sodium valproate. Ann Gen Hosp Psychiatry 2004; 3: 13.
Ross B, Bluml S . Magnetic resonance spectroscopy of the human brain. Anat Rec 2001; 265: 54–84.
Ross BD . Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR Biomed 1991; 4: 59–63.
Brand A, Richter-Landsberg C, Leibfritz D . Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 1993; 15: 289–298.
Kato T, Inubushi T, Kato N . Magnetic resonance spectroscopy in affective disorders. J Neuropsychiatry Clin Neurosci 1998; 10: 133–147.
Videen JS, Michaelis T, Pinto P, Ross BD . Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest 1995; 95: 788–793.
Allison JH, Stewart MA . Reduced brain inositol in lithium-treated rats. Nat New Biol 1971; 233: 267–268.
Hallcher LM, Sherman WR . The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem 1980; 255: 10896–10901.
Manji HK, Bersudsky Y, Chen G, Belmaker RH, Potter WZ . Modulation of protein kinase C isozymes and substrates by lithium: the role of myo-inositol. Neuropsychopharmacology 1996; 15: 370–381.
Berridge MJ, Downes CP, Hanley MR . Neural and developmental actions of lithium: a unifying hypothesis. Cell 1989; 59: 411–419.
Berridge MJ, Downes CP, Hanley MR . Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J 1982; 206: 587–595.
Preece N, Gadian D, Houseman J, Williams S . Lithium-induced modulation of cerebral inositol phosphate metabolism in the rat: a multinuclear magnetic resonance study in vivo. Lithium 1992; 3: 287–297.
Renshaw PF, Summers JJ, Renshaw CE, Hines KG, Leigh Jr JS . Changes in the 31P-NMR spectra of cats receiving lithium chloride systemically. Biol Psychiatry 1986; 21: 694–698.
Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC . Chronic treatment with both lithium and sodium valproate may normalize phosphoinositol cycle activity in bipolar patients. Hum Psychopharmacol 2002; 17: 321–327.
Davanzo P, Yue K, Thomas MA, Belin T, Mintz J, Venkatraman TN et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry 2003; 160: 1442–1452.
Gyulai L, Bolinger L, Leigh Jr JS, Barlow C, Chance B . Phosphorylethanolamine—the major constituent of the phosphomonoester peak observed by 31P-NMR on developing dog brain. FEBS Lett 1984; 178: 137–142.
Pettegrew JW, Kopp SJ, Minshew NJ, Glonek T, Feliksik JM, Tow JP et al. 31P nuclear magnetic resonance studies of phosphoglyceride metabolism in developing and degenerating brain: preliminary observations. J Neuropathol Exp Neurol 1987; 46: 419–430.
Ross B, Michaelis T . Clinical applications of magnetic resonance spectroscopy. Magn Reson Q 1994; 10: 191–247.
Morikawa S, Inubushi T, Kitoh K, Kido C, Nozaki M . Chemical assessment of phospholipid and phosphoenergetic metabolites in regenerating rat liver measured by in vivo and in vitro31P-NMR. Biochim Biophys Acta 1992; 1117: 251–257.
Kato T, Shioiri T, Murashita J, Inubushi T . Phosphorus-31 magnetic resonance spectroscopic observations in 4 cases with anorexia nervosa. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21: 719–724.
Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry 1991; 48: 563–568.
Williamson P, Drost D, Stanley J, Carr T, Morrison S, Merskey H . Localized phosphorus 31 magnetic resonance spectroscopy in chronic schizophrenic patients and normal controls. Arch Gen Psychiatry 1991; 48: 578.
Jensen JE, Drost DJ, Menon RS, Williamson PC . In vivo brain (31)P-MRS: measuring the phospholipid resonances at 4 Tesla from small voxels. NMR Biomed 2002; 15: 338–347.
Yildiz A, Sachs GS, Dorer DJ, Renshaw PF . 31P nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res 2001; 106: 181–191.
Kato T, Shioiri T, Takahashi S, Inubushi T . Measurement of brain phosphoinositide metabolism in bipolar patients using in vivo31P-MRS. J Affect Disord 1991; 22: 185–190.
Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R . Abnormalities of cAMP signaling in affective disorders: implication for pathophysiology and treatment. Bipolar Disord 2000; 2: 27–36.
Hahn CG, Friedman E . Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord 1999; 1: 81–86.
Soares JC, Mallinger AG . Intracellular phosphatidylinositol pathway abnormalities in bipolar disorder patients. Psychopharmacol Bull 1997; 33: 685–691.
Yamawaki S, Kagaya A, Tawara Y, Inagaki M . Intracellular calcium signaling systems in the pathophysiology of affective disorders. Life Sci 1998; 62: 1665–1670.
Simpson PB, Russell JT . Role of mitochondrial Ca2 + regulation in neuronal and glial cell signalling. Brain Res Brain Res Rev 1998; 26: 72–81.
Buchsbaum MS, Wu J, DeLisi LE, Holcomb H, Kessler R, Johnson J et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord 1986; 10: 137–152.
Cohen RM, Semple WE, Gross M, Nordahl TE, King AC, Pickar D et al. Evidence for common alterations in cerebral glucose metabolism in major affective disorders and schizophrenia. Neuropsychopharmacology 1989; 2: 241–254.
Delvenne V, Delecluse F, Hubain PP, Schoutens A, De Maertelaer V, Mendlewicz J . Regional cerebral blood flow in patients with affective disorders. Br J Psychiatry 1990; 157: 359–365.
Drevets WC, Price JL, Simpson Jr JR, Todd RD, Reich T, Vannier M et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997; 386: 824–827.
Baxter Jr LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46: 243–250.
Baxter Jr LR, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE et al. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry 1985; 42: 441–447.
Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry 2001; 49: 97–109.
Migliorelli R, Starkstein SE, Teson A, de Quiros G, Vazquez S, Leiguarda R et al. SPECT findings in patients with primary mania. J Neuropsychiatry Clin Neurosci 1993; 5: 379–383.
Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999; 156: 1986–1988.
Rubin E, Sackeim HA, Prohovnik I, Moeller JR, Schnur DB, Mukherjee S . Regional cerebral blood flow in mood disorders: IV. Comparison of mania and depression. Psychiatry Res 1995; 61: 1–10.
Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry 2000; 48: 1045–1052.
Kato T, Stine OC, McMahon FJ, Crowe RR . Increased levels of a mitochondrial DNA deletion in the brain of patients with bipolar disorder. Biol Psychiatry 1997; 42: 871–875.
Tanaka M, Gong JS, Zhang J, Yoneda M, Yagi K . Mitochondrial genotype associated with longevity. Lancet 1998; 351: 185–186.
Kato T, Kunugi H, Nanko S, Kato N . Association of bipolar disorder with the 5178 polymorphism in mitochondrial DNA. Am J Med Genet 2000; 96: 182–186.
Konradi C, Eaton M, MacDonald ML, Walsh J, Benes FM, Heckers S . Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry 2004; 61: 300–308.
Tarnopolsky MA, Beal MF . Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann Neurol 2001; 49: 561–574.
Rosas HD, Feigin AS, Hersch SM . Using advances in neuroimaging to detect, understand, and monitor disease progression in Huntington's disease. NeuroRx 2004; 1: 263–272.
Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J Neurosci 2000; 20: 4389–4397.
Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. J Neurochem 2003; 85: 1359–1367.
Beal MF . Bioenergetic approaches for neuroprotection in Parkinson's disease. Ann Neurol 2003; 53(Suppl 3): S39–S47; discussion S47–S48.
Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P et al. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. J Neurosci 2004; 24: 5909–5912.
Bolaños JP, Medina JM . Effect of valproate on the metabolism of the central nervous system. Life Sci 1997; 60: 1933–1942.
Modica-Napolitano JS, Lagace CJ, Brennan WA, Aprille JR . Differential effects of typical and atypical neuroleptics on mitochondrial function in vitro. Arch Pharm Res 2003; 26: 951–959.
Balijepalli S, Boyd MR, Ravindranath V . Inhibition of mitochondrial complex I by haloperidol: the role of thiol oxidation. Neuropharmacology 1999; 38: 567–577.
Balijepalli S, Kenchappa RS, Boyd MR, Ravindranath V . Protein thiol oxidation by haloperidol results in inhibition of mitochondrial complex I in brain regions: comparison with atypical antipsychotics. Neurochem Int 2001; 38: 425–435.
Davey GP, Peuchen S, Clark JB . Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 1998; 273: 12753–12757.
Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK . Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol Psychiatry 2004; 9: 734–755.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stork, C., Renshaw, P. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 10, 900–919 (2005). https://doi.org/10.1038/sj.mp.4001711
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001711
Keywords
This article is cited by
-
The metabolic overdrive hypothesis: hyperglycolysis and glutaminolysis in bipolar mania
Molecular Psychiatry (2024)
-
Tremendous Fidelity of Vitamin D3 in Age-related Neurological Disorders
Molecular Neurobiology (2024)
-
Optimization of differential filtration-based mitochondrial isolation for mitochondrial transplant to cerebral organoids
Stem Cell Research & Therapy (2023)
-
Antidepressants that increase mitochondrial energetics may elevate risk of treatment-emergent mania
Molecular Psychiatry (2023)
-
Impaired neural stress resistance and loss of REST in bipolar disorder
Molecular Psychiatry (2023)