Abstract
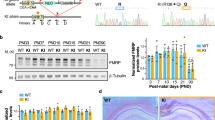
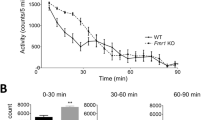
Fragile X syndrome (FXS) is the most common single gene (FMR1) disorder affecting cognitive and behavioral function in humans. This syndrome is characterized by a cluster of abnormalities including lower IQ, attention deficits, impairments in adaptive behavior and increased incidence of autism. Here, we show that young males with FXS have profound deficits in prepulse inhibition (PPI), a basic marker of sensorimotor gating that has been extensively studied in rodents. Importantly, the magnitude of the PPI impairments in the fragile X children predicted the severity of their IQ, attention, adaptive behavior and autistic phenotypes. Additionally, these measures were highly correlated with each other, suggesting that a shared mechanism underlies this complex phenotypic cluster. Studies in Fmr1-knockout mice also revealed sensorimotor gating and learning abnormalities. However, PPI and learning were enhanced rather than reduced in the mutants. Therefore, these data show that mutations of the FMR1 gene impact equivalent processes in both humans and mice. However, since these phenotypic changes are opposite in direction, they also suggest that murine compensatory mechanisms following loss of FMR1 function differ from those in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Turner G, Webb T, Wake S, Robinson H . Prevalence of fragile × syndrome. Am J Med Genet 1996; 64: 196–197.
Verkerk AJ et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 1991; 65: 905–914.
Hagerman RJ, Hagerman PJ . Fragile X Syndrome: Diagnosis, Treatmen, and Research, Vol xii Johns Hopkins University Press: Baltimore, 2002 540pp.
Kooy RF, Willemsen R, Oostra BA . Fragile X syndrome at the turn of the century. Mol Med Today 2000; 6: 193–198.
O'Donnell WT, Warren ST . A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci 2002; 25: 315–338.
Greenough WT et al. Synaptic regulation of protein synthesis and the fragile X protein. Proc Natl Acad Sci (USA) 2001; 98: 7101–7106.
Bakker CE et al. Fmr1 knockout mice—a model to study fragile X mental retardation. Cell 1994; 78: 23–33.
Peier AM et al. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet 2000; 9: 1145–1159.
Nielsen DM, Derber WJ, McClellan DA, Crnic LS . Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile X syndrome. Brain Res 2002; 927: 8–17.
Miller LJ et al. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet 1999; 83: 268–279.
Geyer MA, McIlwain KL, Paylor R . Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry 2002; 7: 1039–1053.
Sparrow SS, Balla D, Cicchetti DV . Vineland Adaptive Behavior Scales. American Guidance Service: Circle Pines, MN, 1984.
Achenbach TM . Manual of the Child Behavior Checklist. University of Vermont: Burlington, VT, 1991.
Berument SK, Rutter M, Lord C, Pickles A, Bailey A . Autism screening questionnaire: diagnostic validity. Br J Psychiatry 1999; 175: 444–451.
Kaufman AS, Kaufman NL . Kaufman Brief Intelligence Test. American Guidance Service: Circle Pines, MN, 1990.
Ornitz EM et al. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biol Psychiatry 1999; 45: 1455–1466.
Carvalho OM, Silva AJ, Balleine BW . Evidence of selective learning deficits on tests of Pavlovian and instrumental conditioning on alpha-CaMKII-T286 mutant mice. Int J Comp Psychol 2001; 14: 161–174.
Dickinson A, Squire S, Varga Z, Smith JW . Omission learning after instrumental pretraining. Quart J Exp Psychol Comp Physiol Psychol 1998; 51B: 271–286.
Hutchison KE, Swift R . Effect of D-amphetamine on prepulse inhibition of the startle reflex in humans. Psychopharmacology (Berl) 1999; 143: 394–400.
Swerdlow NR et al. Dopamine agonist effects on startle and sensorimotor gating in normal male subjects: time course studies. Psychopharmacology (Berl) 2002; 161: 189–201.
Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E . Effects of bromocriptine and haloperidol on prepulse inhibition of the acoustic startle response in man. J Psychopharmacol 1998; 12: 239–245.
Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E . Effects of clonidine and diazepam on prepulse inhibition of the acoustic startle response and the N1/P2 auditory evoked potential in man. J Psychopharmacol 2001; 15: 237–242.
Schachinger H, Muller BU, Strobel W, Langewitz W, Ritz R . Midazolam effects on prepulse inhibition of the acoustic blink reflex. Br J Clin Pharmacol 1999; 47: 421–426.
Mackeprang T, Kristiansen KT, Glenthoj BY . Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol Psychiatry 2002; 52: 863–873.
Graham SJ, Langley RW, Bradshaw CM, Szabadi E . Effects of haloperidol and clozapine on prepulse inhibition of the acoustic startle response and the N1/P2 auditory evoked potential in man. J Psychopharmacol 2001; 15: 243–250.
Pouretemad HR, Thompson PJ, Fenwick PB . Sensorimotor gating in patients with non-epileptic seizures. Epilepsy Res 1998; 31: 1–12.
Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR . Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 2001; 156: 117–154.
Braff DL, Geyer MA, Swerdlow NR . Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001; 156: 234–258.
Chen L, Toth M . Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 2001; 103: 1043–1050.
Van Dam D et al. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav Brain Res 2000; 117: 127–136.
Dobkin C et al. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience 2000; 100: 423–429.
Qin M, Kang J, Smith CB . Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation. Proc Natl Acad Sci (USA) 2002; 99: 15758–15763.
D'Hooge R et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience 1997; 76: 367–376.
Fisch GS, Hao HK, Bakker C, Oostra BA . Learning and memory in the FMR1 knockout mouse. Am J Med Genet 1999; 84: 277–282.
Squire LR, Knowlton B, Musen G . The structure and organization of memory. Annu Rev Psychol 1993; 44: 453–495.
Balleine BW, Dickinson A . Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 1998; 37: 407–419.
Watase K, Zoghbi HY . Modelling brain diseases in mice: the challenges of design and analysis. Nat Rev Genet 2003; 4: 296–307.
Jin P, Warren ST . New insights into fragile X syndrome: from molecules to neurobehaviors. Trends Biochem Sci 2003; 28: 152–158.
Feng Y et al. FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell 1997; 1: 109–118.
Tamanini F et al. Differential expression of FMR1, FXR1 and FXR2 proteins in human brain and testis. Hum Mol Genet 1997; 6: 1315–1322.
Bontekoe CJ et al. Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet 2002; 11: 487–498.
Acknowledgements
These studies were supported by FRAXA Research Foundation grants to PWF and AJS, and to EMD and EMO, and by support from the Virginia Friedhofer Charitable Trust to EMO. We thank Sheena Josselyn and Anna Matynia for comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Frankland, P., Wang, Y., Rosner, B. et al. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry 9, 417–425 (2004). https://doi.org/10.1038/sj.mp.4001432
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001432
Keywords
This article is cited by
-
Endophenotype trait domains for advancing gene discovery in autism spectrum disorder
Journal of Neurodevelopmental Disorders (2023)
-
Developmental Impairments of Synaptic Refinement in the Thalamus of a Mouse Model of Fragile X Syndrome
Neuroscience Bulletin (2023)
-
Sensory Symptoms and Signs of Hyperarousal in Individuals with Fragile X Syndrome: Findings from the FORWARD Registry and Database Multisite Study
Journal of Autism and Developmental Disorders (2023)
-
Auditory processing in rodent models of autism: a systematic review
Journal of Neurodevelopmental Disorders (2022)
-
Electroretinography and contrast sensitivity, complementary translational biomarkers of sensory deficits in the visual system of individuals with fragile X syndrome
Journal of Neurodevelopmental Disorders (2021)