Abstract
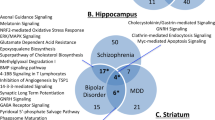
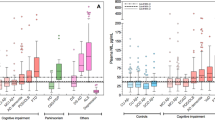
We previously demonstrated that apolipoprotein D (apoD) levels are elevated in the dorsolateral prefrontal cortex and caudate obtained postmortem from subjects with schizophrenia and bipolar disorder compared to controls, suggesting a focal compensatory response to neuropathology associated with psychiatric disorders. We have now extended those studies by measuring apoD protein levels in additional brain regions from post-mortem samples of schizophrenic and bipolar disorder subjects using an enzyme-linked immunosorbent assay. Increased apoD levels were observed in the lateral prefrontal cortex (Brodmann Area 46) in both schizophrenia (46%) and bipolar disorder (111%), and in the orbitofrontal cortex (Brodmann Area 11) (44.3 and 37.9% for schizophrenia and bipolar disorder, respectively). However, differences between the disease groups were observed in other brain regions. In subjects with schizophrenia, but not bipolar disorder, apoD levels were significantly elevated in the amygdala (42.8%) and thalamus (31.7%), while in bipolar disorder, but not schizophrenia, additional increases were detected in the parietal cortex (Brodmann Area 40; 123%) and the cingulate cortex (Brodmann Area 24; 57.7%). These data demonstrate that there is anatomical overlap in the pathophysiologies of schizophrenia and bipolar disorder, as well as areas of pathology that distinguish the two disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
McConathy WJ, Alaupovic P . Isolation and partial characterization of apolipoprotein D: a new protein moiety of the human plasma lipoprotein system. FEBS Lett 1973; 37: 178–182.
Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E et al. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem 1986; 261.
Vogt M, Skerra A . Bacterially produced apolipoprotein D binds progesterone and arachidonic acid, but not bilirubin or E-3M2H. J Mol Recognition 2001; 14: 79–86.
Morais-Cabral JH, Atkins GL, Sanchez LM, Lopez-Boado YS, Lopez-Otin C, Sawyer L . Arachidonic acid binds to apoliprotein D: implications for the protein's function. FEBS Lett 1995; 366: 53–56.
Dilley WG, Haagensen DE, Cox CE, Wells SA . Immunologic and steroid binding properties of CDFP-24 protein isolated from human breast gross cystic disease fluid. Breast Cancer Res Treat 1990; 16: 253–260.
Lea OA . Binding properties of progesterone-binding cyst protein PBCP. Steroids 1988; 52: 337–338.
Zeng C, Spielman AI, Vowels BR, Leyden JJ, Biemann K, Preti G . A human axillary odorant is carried by apolipoprotein D. Proc Natl Acad Sci USA 1996; 93: 6626–30.
Ong WY, He Y, Suresh S, Patel SC . Differential expression of apoliprotein D and apoliprotein E in the kainic acid-lesioned rat hippocampus. Neuroscience 1997; 79: 359–367.
Montpied P, de Bock F, Lerner-Natoli M, Bockaert J, Rondouin G . Hippocampal alterations of apolipoprotein E and D mRNA levels in vivo and in vitro following kainate excitotoxicity. Epilepsy Res 1999; 35: 135–146.
Franz G, Reindl M, Patel SC, Beer R, Unterrichter I, Berger T et al. Increased expression of apolipoprotein D following experimental traumatic brain injury. J Neurochem 1999; 73: 1615–1625.
Yoshida K, Cleaveland ES, Nagle JW, French S, Yaswen L, Ohshima T et al. Molecular cloning of the mouse apolipoprotein D gene and its upregulated expression in Niemann–Pick disease type C mouse model. DNA Cell Biol 1996; 15: 873–882.
Suresh S, Yan Z, Patel RC, Patel YC, Patel SC . Cellular cholesterol storage in the Niemann–Pick disease type C mouse is associated with increased expression and defective processing of apolipoprotein D. J Neurochem 1998; 70: 242–251.
Terrisse L, Poirier J, Bertrand P, Merched A, Visvikis S, Siest G et al. Increased levels of apolipoprotein D in cerebrospinal fluid and hippocampus of Alzheimer's patients. J Neurochem 1998; 71: 1643–1650.
Kalman J, McConathy W, Araoz C, Kasa P, Lacko A . Apolipoprotein D in the aging brain and in Alzheimer's dementia. Neurol Res 2000; 22: 330–336.
Belloir B, Kovari E, Surini-Demiri M, Savioz A . Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J Neurosci Res 2001; 64: 61–69.
Reindl M, Knipping G, Wicher I, Dilitz E, Egg R, Deisenhammer F et al. Increased intrathecal production of apolipoproten D in multiple sclerosis. J Neuroimmunol 2001; 119: 327–332.
Thomas EA, Dean B, Pavey G, Sutcliffe JG . Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA 2001; 98: 4066–4071.
Diagnostic and Statistical Manual of Mental Disorders, IV, 4th edn. American Psychiatric Association: Washington, D C, 1994.
Keks NA, Hill C, Opeskin K, Copolov DL, Dean B . Psychiatric diagnosis after death: the problems of accurate diagnosis from the case history review and relative interviews. In: DB, Kleinman JE, Hyde TM (eds). Using CNS Tissue in Psychiatric Research. Harwood Academic Press: Amsterdam, 1999, pp 19–37.
Berrettini WH . Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry 2000; 48: 531–538.
Bunney WE, Bunney BG . Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Brain Res Rev 2000; 31: 138–146.
Goldman-Rakic PS, Seleman LD . Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr Bull 1997; 23: 437–458.
Franzen G, Ingvar DH . Absence of activation in frontal structures during psychological testing of chronic schizophrenics. J Neurol Neurosurg Psychiatry 1975; 38: 1027–1032.
Weinberger DR, Berman KF, Zec RF . Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry 1986; 43: 114–134.
Berman KF, Weinberger DR . The prefrontal cortex in schizophrenia and other neuropsychiatric diseases: in vivo physiological correlates of cognitive deficits. Prog Brain Res 1990; 85: 521–536.
Liddle PF, Morris DL . Schizophrenia syndromes and frontal lobe performance. Br J Psychiatry 1991; 158: 340–345.
Drevets WC, Ongur D, Price JL . Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol Psychiatry 1998; 3: 220–226.
Knable MB . Schizophrenia and bipolar disorder: findings from studies of the Stanley Foundation Brain Collection. Schizophr Res 1999; 39: 149–152.
Freedman M, Black S, Ebert P, Binns M . Orbitofrontal function, object alternation and perseveration. Cereb Cortex 1998; 8: 18–27.
Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Magnotta V . Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry 2000; 48: 110–119.
Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS . Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry 1999; 45: 1597–1605.
Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999; 156: 1986–1988.
Joseph R . Frontal lobe psychopathology: mania, depression, confabulation, catatonia, perseveration, obsessive compulsions, and schizophrenia. Psychiatry 1999; 62: 138–172.
Yudofsky SC, Hales RE . The American Psychiatric Press Textbook of Neuropsychiatry, 3rd edn. American Psychiatric Press: Washington, DC, 1997.
Barr WB . Schizophrenia and attention deficit disorder. Two complex disorders of attention. Ann N Y Acad Sci 2001; 931: 239–250.
Pliszka SR . Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: an overview. J Clin Psychiatry 1998; 7: 50–58.
Eastwood SL, Harrison PJ . Synaptic pathology in the anterior cingulate cortex in schizophrenia and mood disorders. A review and a Western blot study of synaptophysin, GAP-43 and the complexins. Brain Res Bull 2001; 55: 569–578.
Benes FM, Vincent SL, Todtenkopf M . The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry 2001; 50: 395–406.
Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A et al. PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153: 191–199.
Andreasen NC, Flashman L, Flaum M, Arndt S, Swayze Vn, O'Leary DS et al. Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. JAMA 1994; 272: 1763–1769.
Thune JJ, Pakkenberg B . Stereological studies of the schizophrenic brain. Brain Res Brain Res Rev 2000; 31: 200–204.
Aggleton JP . The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci 1993; 16: 328–333.
Fudge JL, Powers JM, Haber SN, Caine ED . Considering the role of the amygdala in psychotic illness: a clinicopathological correlation. J Neuropsychiatry Clin Neurosci 1998; 10: 383–394.
Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK . Mapping of grey matter changes in schizophrenia. Schizophr Res 1999; 35: 1–14.
Tamminga CA, Vogel M, Gao X, Lahti AC, Holcomb HH . The limbic cortex in schizophrenia: focus on the anterior cingulate. Brain Res Brain Res Rev 2000; 31: 364–370.
Cotter D, Mackay D, Landau S, Kerwin R, Everall I . Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 2001; 58: 545–553.
Carter CS, Mintun M, Nichols T, Cohen JD . Anterior cinuglate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997; 154: 1670–1675.
Harrison PJ . The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain 1999; 122: 593–624.
Thomas EA, Danielson PE, Nelson PA, Pribyl TM, Hilbush BS, Hasel KW et al. Clozapine increases apolipoprotein D expression in rodent brain: towards a mechanism for neuroleptic pharmacotherapy. J Neurochem 2001; 76: 789–796.
Thomas EA, Sautkulis LN, Criado JR, Games D, Sutcliffe JG . Apolipoprotein D mRNA expression is elevated in PDAPP transgenic mice. J Neurochem 2001; 79: 1059–1064.
Lechner M, Wojnar P, Redl B . Human tear lipocalin acts as an oxidative-stress-induced scavenger of potentially harmful lipid peroxidation products in a cell culture system. Biochem J 2001; 356: 129–135.
Yao JK, Reddy RD, van Kammen DP . Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs 2001; 15: 287–310.
Horrobin DF, Bennet CN . New gene targets related to schizophrenia and other psychiatric disorders: enzymes, binding proteins and transport proteins involved in phospholipid and fatty acid metabolism. Prostaglandins Leukot Essent Fatty Acids 1999; 60: 141–167.
Acknowledgements
This work was supported in part by NIH Grant GM32355, NHMRC Grant 193299 and Digital Gene Technologies. We thank Geoffrey Pavey for neuroanatomical dissections. We thank Nicholas Keks and Christine Hill for case-history review and subsequent diagnosis of the schizophrenic and bipolar subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomas, E., Dean, B., Scarr, E. et al. Differences in neuroanatomical sites of apoD elevation discriminate between schizophrenia and bipolar disorder. Mol Psychiatry 8, 167–175 (2003). https://doi.org/10.1038/sj.mp.4001223
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001223
Keywords
This article is cited by
-
Enrichment of apolipoprotein A-IV and apolipoprotein D in the HDL proteome is associated with HDL functions in diabetic kidney disease without dialysis
Lipids in Health and Disease (2020)
-
Global proteomic profiling reveals altered proteomic signature in schizophrenia serum
Molecular Psychiatry (2010)
-
Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues
Molecular Psychiatry (2008)
-
Decreased levels of apolipoprotein A-I in plasma of schizophrenic patients
Journal of Neural Transmission (2007)
-
The boundaries of schizophrenia: Overlap with bipolar disorders
Current Psychosis and Therapeutics Reports (2004)