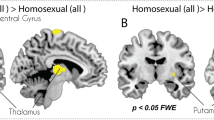
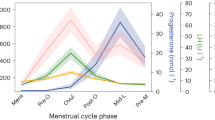
Abstract
We briefly review the technique of functional brain imaging and its application in the assessment of the sexual response in men and women.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kwong KK, Belliveau JW, Chesler DA, Chesler DA, Goldberg IE, Weisskoff RM et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA 1992; 89: 5675–5679.
Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 1992; 89: 5951–5955.
Stoleru S, Gregoire MC, Gerard D, Decety J, Zafarge E, Cinotti Z et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav 1999; 28: 1–21.
Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW . Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology 2001; 57: 1189–1194.
Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 2002; 16: 1–13.
Hamann S, Herman RA, Nolan CL, Wallen K . Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci 2004; 7: 411–416.
Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A . An fMRI study of simple ethical decision-making. NeuroReport 2003; 14: 1215–1219.
Moll J, de Oliveira-Souza R, Bramati IE, Grafman J . Functional networks in emotional moral and nonmoral social judgments. Neuroimage 2002; 16(3 Part 1): 696–703.
Montorsi F, Perani D, Anchisi D, Salonia A, Scifo P, Rigoroli P et al. Brain activation patterns during video sexual stimulation following the administration of apomorphine: results of a placebo-controlled study. Eur Urol 2003; 43: 405–411.
Komisaruk BR, Whipple B, Crawford A, Liu WC, Kalnin A, Mosier K . Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res 2004; 1024: 77–88.
Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 2005; 26: 1086–1096.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maravilla, K., Yang, C. Sex and the brain: the role of fMRI for assessment of sexual function and response. Int J Impot Res 19, 25–29 (2007). https://doi.org/10.1038/sj.ijir.3901493
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901493
Keywords
This article is cited by
-
The relation between sexual interest and personality characteristics in men: an eye-tracking study
International Journal of Impotence Research (2010)