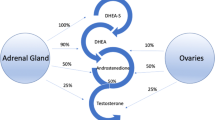
Abstract
Male hypogonadism is associated with potentially distressing adverse effects on diverse organs and tissues. These include sexual dysfunction, particularly diminished libido, as well as mood disturbances, reduced lean body mass, and increased adipose-tissue mass. A wide range of effective and well-tolerated options exists. These include relatively noninvasive therapies, such as testosterone (T) gels and T patches; slightly more invasive treatments, such as the T buccal system; and invasive therapies, such as intramuscular T injections and subcutaneous depot implants (T pellets). Testosterone replacement therapy (TRT) can be individualized to enhance patient health and well-being. Screening and ongoing monitoring are necessary to ensure both the efficacy and safety of TRT, particularly prostate safety. Investigational agents, including selective androgen receptor modulators, may offer new pharmacodynamic and/or pharmacokinetic properties that enhance outcomes of TRT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Arver S . Advances in methods of testosterone replacement therapy. In: Wu CW (ed). Testosterone Replacement Therapy. J Endocrinol Ltd: Bristol, UK, 1996, pp 31–47.
Lue TF, Giuliano F, Montorsi F, Rosen RC, Andersson K-E, Althof S et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 2004; 1: 6–23.
Shabsigh R, Kaufman JM, Steidle C, Padma-Nathan H . Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 2004; 172: 658–663.
Mulhall JP, Valenzuela R, Aviv N, Parker M . Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63: 348–353.
American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients: 2002 update. Endocr Pract 2002; 8: 440–456.
Solvay Pharmaceuticals. AndroGel® (testosterone gel) 1% US prescribing information. Available at http://www.solvaypharmaceuticals-us.com/static/wma/pdf/1/3/1/4/androgel_prescribing.pdf. Accessed October 12, 2004.
Auxilium Pharmaceuticals. Testim® 1% (testosterone gel) US prescribing information. Available at http://www.auxilium.com/PrescribingInformation-04Sep2003.pdf. Accessed October 12, 2004.
Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 4500–4510.
Marbury T, Hamill E, Bachand R, Sebree T, Smith T . Evaluation of the pharmacokinetic profiles of the new testosterone topical gel formulation, Testim, compared to AndroGel. Biopharm Drug Dispos 2003; 24: 115–120.
Seftel AD, Mack RJ, Secrest AR, Smith TM . Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. J Androl 2004; 25: 963–972.
Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R . AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab 2003; 88: 2673–2681.
Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G et al. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab 2000; 85: 2839–2853.
Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G et al. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Testosterone Gel Study Group. Clin Endocrinol (Oxf) 2001; 54: 739–750.
Pope Jr HG, Cohane GH, Kanayama G, Siegel AJ, Hudson JI . Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003; 160: 105–111.
De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, Kaplan SH et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care 2002; 25: 284–291.
Penson DF, Latini DM, Lubeck DP, Wallace KL, Henning JM, Lue TF . Do impotent men with diabetes have more severe erectile dysfunction and worse quality of life than the general population of impotent patients? Results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction (ExCEED) database. Diabetes Care 2003; 26: 1093–1099.
Park K, Ku JH, Kim SW, Paick J-S . Risk factors in predicting a poor response to sildenafil citrate in elderly men with erectile dysfunction. BJU Int 2005; 95: 366–370.
Greenstein A, Mabjeesh NJ, Sofer M, Kaver I, Matzkin H, Chen J . Does sildenafil combined with testosterone gel improve erectile dysfunction in hypogonadal men in whom testosterone supplement therapy alone failed? J Urol 2005; 173: 530–532.
Orengo CA, Fullerton L, Kunik ME . Safety and efficacy of testosterone gel 1% augmentation in depressed men with partial response to antidepressant therapy. J Geriatr Psychiatry Neurol 2005; 18: 20–24.
Rolf C, Knie U, Lemmnitz G, Nieschlag E . Interpersonal testosterone transfer after topical application of a newly developed testosterone gel preparation. Clin Endocrinol (Oxf) 2002; 56: 637–641.
Siegfried D . Red Book: Pharmacy's Fundamental Reference 2004 Edition. Thomson PDR: Montvale, NJ.
Brocks DR, Meikle AW, Boike SC, Mazer NA, Zariffa N, Audet PR et al. Pharmacokinetics of testosterone in hypogonadal men after transdermal delivery: influence of dose. J Clin Pharmacol 1996; 36: 732–739.
Wilson DE, Meikle AW, Boike SC, Fairless AJ, Etheredge RC, Jorkasky DK . Bioequivalence assessment of a single 5 mg/day testosterone transdermal system versus two 2.5 mg/day systems in hypogonadal men. J Clin Pharmacol 1998; 38: 54–59.
Meikle AW, Arver S, Dobs AS, Sanders SW, Rajaram L, Mazer NA . Pharmacokinetics and metabolism of a permeation-enhanced testosterone transdermal system in hypogonadal men: influence of application site – a clinical research center study. J Clin Endocrinol Metab 1996; 81: 1832–1840.
Jain P, Rademaker AW, McVary KT . Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164: 371–375.
Arver S, Dobs AS, Meikle AW, Allen RP, Sanders SW, Mazer NA . Improvement of sexual function in testosterone deficient men treated for 1 y with a permeation enhanced testosterone transdermal system. J Urol 1996; 155: 1604–1608.
Arver S, Dobs AS, Meikle AW, Caramelli KE, Rajaram L, Sanders SW et al. Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men. Clin Endocrinol (Oxf) 1997; 47: 727–737.
Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG . Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci 2001; 56: M266–M272.
Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH et al. Effect of testosterone treatment on bone mineral density in men over 65 y of age. J Clin Endocrinol Metab 1999; 84: 1966–1972.
Monga M, Kostelec M, Kamarei M . Patient satisfaction with testosterone supplementation for the treatment of erectile dysfunction. Arch Androl 2002; 48: 433–442.
Watson Laboratories. Androderm® (testosterone transdermal system) US prescribing information. Available at http://www.watsonpharma.com/data_streamasp?product_group=4&p=pi&top=02001. Accessed October 12, 2004.
Jordan Jr WP . Allergy and topical irritation associated with transdermal testosterone administration: a comparison of scrotal and nonscrotal transdermal systems. Am J Contact Dermatol 1997; 8: 108–113.
Parker S, Armitage M . Experience with transdermal testosterone replacement therapy for hypogonadal men. Clin Endocrinol (Oxf) 1999; 50: 57–62.
Columbia Laboratories. Striant® (testosterone buccal system) US prescribing information. Available at http://www.columbialabs.com/Strian/Striant_Full_Prescribing_info_print.htm. Accessed October 12, 2004.
Dobs AS, Hoover DR, Chen MC, Allen R . Pharmacokinetic characteristics, efficacy, and safety of buccal testosterone in hypogonadal males: a pilot study. J Clin Endocrinol Metab 1998; 83: 33–39.
Dobs AS, Matsumoto AM, Wang C, Kipnes MS . Short-term pharmacokinetic comparison of a novel testosterone buccal system and a testosterone gel in testosterone deficient men. Curr Med Res Opin 2004; 20: 729–738.
Korbonits M, Slawik M, Cullen D, Ross RJ, Stalla G, Schneider H et al. A comparison of a novel testosterone bioadhesive buccal system, Striant, with a testosterone adhesive patch in hypogonadal males. J Clin Endocrinol Metab 2004; 89: 2039–2043.
O'Carroll R, Bancroft J . Testosterone therapy for low sexual interest and erectile dysfunction in men: a controlled study. Br J Psychiatry 1984; 145: 146–151.
Morales A, Johnston B, Heaton JW, Clark A . Oral androgens in the treatment of hypogonadal impotent men. J Urol 1994; 152: 1115–1118.
Skakkebaek NE, Bancroft J, Davidson DW, Warner P . Androgen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled study. Clin Endocrinol (Oxf) 1981; 14: 49–61.
Morales A, Johnston B, Heaton JP, Lundie M . Testosterone supplementation for hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol 1997; 157: 849–854.
Mullen JO, Juchau MR, Fouts JR . Studies of 3,4-benzpyrene, 3-methylcholanthrene, chlordane, and methyltestosterone as stimulators of hepatic microsomal enzyme systems in the rat. Biochem Pharmacol 1966; 15: 137–144.
Bird DR, Vowles KD . Liver damage from long-term methyltestosterone. Lancet 1977; 2: 400–401.
Bagheri SA, Boyer JL . Peliosis hepatis associated with androgenic-anabolic steroid therapy: a severe form of hepatic injury. Ann Intern Med 1974; 81: 610–618.
Boyd PR, Mark GJ . Multiple hepatic adenomas and a hepatocellular carcinoma in a man on oral methyl testosterone for eleven years. Cancer 1977; 40: 1765–1770.
Conway AJ, Boylan LM, Howe C, Ross G, Handelsman DJ . Randomized clinical trial of testosterone replacement therapy in hypogonadal men. Int J Androl 1988; 11: 247–264.
Gooren LJ . A ten-year safety study of the oral androgen testosterone undecanoate. J Androl 1994; 15: 212–215.
Conway AJ, Handelsman DJ, Lording DW, Stuckey B, Zajac JD . Use, misuse and abuse of androgens: The Endocrine Society of Australia consensus guidelines for androgen prescribing. Med J Aust 2000; 172: 220–224.
Pfizer Inc. Testosterone cypionate (Depo-Testosterone) US prescribing information. Available at http://www.pfizer.com/download/uspi_depo_testosterone.pdf. Accessed October 12, 2004.
Morales A . Testosterone replacement: when is there a role? Int J Impot Res 2000; 12 (Suppl 4): S112–S118.
Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA . Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84: 3469–3478.
Zhang GY, Gu YQ, Wang XH, Cui YG, Bremner WJ . A pharmacokinetic study of injectable testosterone undecanoate in hypogonadal men. J Androl 1998; 19: 761–768.
Salmimies P, Kockott G, Pirke KM, Vogt HJ, Schill WB . Effects of testosterone replacement on sexual behavior in hypogonadal men. Arch Sex Behav 1982; 11: 345–353.
Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V et al. Testosterone replacement therapy improves mood in hypogonadal men: a clinical research center study. J Clin Endocrinol Metab 1996; 81: 3578–3583.
Nieschlag E, Buchter D, von Eckardstein S, Abshagen K, Simoni M, Behre HM . Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men. Clin Endocrinol (Oxf) 1999; 51: 757–763.
Davidson JM, Camargo CA, Smith ER . Effects of androgen on sexual behavior in hypogonadal men. J Clin Endocrinol Metab 1979; 48: 955–958.
Behre HM, Abshagen K, Oettel M, Hubler D, Nieschlag E . Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies. Eur J Endocrinol 1999; 140: 414–419.
Grinspoon S, Corcoran C, Askari H, Schoenfeld D, Wolf L, Burrows B et al. Effects of androgen administration in men with the AIDS wasting syndrome: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998; 129: 18–26.
von Eckardstein S, Nieschlag E . Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. J Androl 2002; 23: 419–425.
BTG Pharmaceuticals. Delatestryl® US prescribing information. Available at http://www.delatestryl.com/prescribing_body.htm. Accessed October 12, 2004.
Carrasco D, Prieto M, Pallardo L, Moll JL, Cruz JM, Muñoz C et al. Multiple hepatic adenomas after long-term therapy with testosterone enanthate: review of the literature. J Hepatol 1985; 1: 573–578.
Handelsman DJ, Mackey MA, Howe C, Turner L, Conway AJ . An analysis of testosterone implants for androgen replacement therapy. Clin Endocrinol (Oxf) 1997; 47: 311–316.
Jockenhövel F, Vogel E, Kreutzer M, Reinhardt W, Lederbogen S, Reinwein D . Pharmacokinetics and pharmacodynamics of subcutaneous testosterone implants in hypogonadal men. Clin Endocrinol (Oxf) 1996; 45: 61–71.
Handelsman DJ, Conway AJ, Boylan LM . Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J Clin Endocrinol Metab 1990; 71: 216–222.
Kelleher S, Turner L, Howe C, Conway AJ, Handelsman DJ . Extrusion of testosterone pellets: a randomized controlled clinical study. Clin Endocrinol (Oxf) 1999; 51: 469–471.
Kelleher S, Conway AJ, Handelsman DJ . A randomised controlled clinical trial of antibiotic impregnation of testosterone pellet implants to reduce extrusion rate. Eur J Endocrinol 2002; 146: 513–518.
Rhoden EL, Morgentaler A . Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350: 482–492.
McGriff NJ, Csako G, Kabbani M, Diep L, Chrousos GP, Pucino F . Treatment options for a patient experiencing pruritic rash associated with transdermal testosterone: a review of the literature. Pharmacotherapy 2001; 21: 1425–1435.
Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR . Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl 2003; 24: 299–311.
Snyder PJ . Hypogonadism in elderly men: what to do until the evidence comes. N Engl J Med 2004; 350: 440–442.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 2004; 291: 1701–1712.
Strickler RC . Women's Health Initiative results: a glass more empty than full. Fertil Steril 2003; 80: 488–490.
Machens K, Schmidt-Gollwitzer K . Issues to debate on the Women's Health Initiative (WHI) study. Hormone replacement therapy: an epidemiological dilemma? Hum Reprod 2003; 18: 1992–1999.
Douglas TH, Connelly RR, McLeod DG, Erickson SJ, Barren III R, Murphy GP . Effect of exogenous testosterone replacement on prostate-specific antigen and prostate-specific membrane antigen levels in hypogonadal men. J Surg Oncol 1995; 59: 246–250.
Behre HM, Bohmeyer J, Nieschlag E . Prostate volume in testosterone-treated and untreated hypogonadal men in comparison to age-matched normal controls. Clin Endocrinol (Oxf) 1994; 40: 341–349.
Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 2000; 85: 2670–2677.
Hajjar RR, Kaiser FE, Morley JE . Outcomes of long-term testosterone replacement in older hypogonadal males: a retrospective analysis. J Clin Endocrinol Metab 1997; 182: 3793–3798.
Sih R, Morley JE, Kaiser FE, Perry III HM, Patrick P, Ross C . Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab 1997; 82: 1661–1667.
Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N et al. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol 2003; 63: 211–223.
Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y et al. Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther 2003; 304: 1334–1340.
Berrevoets CA, Umar A, Brinkmann AO . Antiandrogens: selective androgen receptor modulators. Mol Cell Endocrinol 2002; 198: 97–103.
Negro-Vilar A . Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab 1999; 84: 3459–3462.
Swerdloff RS, Wang C . Dihydrotestosterone: a rationale for its use as a non-aromatizable androgen replacement therapeutic agent. Baillieres Clin Endocrinol Metab 1998; 12: 501–506.
Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J et al. Differential effects on bone of estrogen receptor α and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA 2003; 100: 13573–13578.
Wang C, Iranmanesh A, Berman N, McDonald V, Steiner B, Ziel F et al. Comparative pharmacokinetics of three doses of percutaneous dihydrotestosterone gel in healthy elderly men: a clinical research center study. J Clin Endocrinol Metab 1998; 83: 2749–2757.
Carson III C, Rittmaster R . The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003; 61: 2–7.
Nunlist EH, Dozmorov I, Tang Y, Cowan R, Centola M, Lin HK . Partitioning of 5α-dihydrotestosterone and 5α-androstane-3α, 17β-diol activated pathways for stimulating human prostate cancer LNCaP cell proliferation. J Steroid Biochem Mol Biol 2004; 91: 157–170.
McCulloch DR, Akl P, Samaratunga H, Herington AC, Odorico DM . Expression of the disintegrin metalloprotease, ADAM-10, in prostate cancer and its regulation by dihydrotestosterone, insulin-like growth factor I, and epidermal growth factor in the prostate cancer cell model LNCaP. Clin Cancer Res 2004; 10: 314–323.
Hajdinjak T, Zagradisnik B . Prostate cancer and polymorphism D85Y in gene for dihydrotestosterone degrading enzyme UGT2B15: frequency of DD homozygotes increases with Gleason Score. Prostate 2004; 59: 436–439.
Marhefka CA, Gao W, Chung K, Kim J, He Y, Yin D et al. Design, synthesis, and biological characterization of metabolically stable selective androgen receptor modulators. J Med Chem 2004; 47: 993–998.
Edwards JP, Higuchi RI, Winn DT, Pooley CL, Caferro TR, Hamann LG et al. Nonsteroidal androgen receptor agonists based on 4-(trifluoromethyl)-2H-pyrano[3,2-g]quinolin-2-one. Bioorg Med Chem Lett 1999; 9: 1003–1008.
Edwards JP, West SJ, Pooley CL, Marschke KB, Farmer LJ, Jones TK . New nonsteroidal androgen receptor modulators based on 4-(trifluoromethyl)-2(1H)-pyrrolidino[3,2-g] quinolinone. Bioorg Med Chem Lett 1998; 8: 745–750.
Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T et al. Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol Pharm Bull 2003; 26: 1563–1569.
Anderson RA, Martin CW, Kung AW, Everington D, Pun TC, Tan KC et al. 7α-methyl-19-nortestosterone maintains sexual behavior and mood in hypogonadal men. J Clin Endocrinol Metab 1999; 84: 3556–3562.
Suvisaari J, Moo-Young A, Juhakoski A, Elomaa K, Saleh SI, Lahteenmaki P . Pharmacokinetics of 7α-methyl-19-nortestosterone (MENT) delivery using subdermal implants in healthy men. Contraception 1999; 60: 299–303.
O'Carroll R, Shapiro C, Bancroft J . Androgens, behaviour and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol (Oxf) 1985; 23: 527–538.
Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ . A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl 1992; 13: 297–304.
Burris AS, Ewing LL, Sherins RJ . Initial trial of slow-release testosterone microspheres in hypogonadal men. Fertil Steril 1988; 50: 493–497.
Bhasin S, Swerdloff RS, Steiner B, Peterson MA, Meridores T, Galmirini M et al. A biodegradable testosterone microcapsule formulation provides uniform eugonadal levels of testosterone for 10–11 weeks in hypogonadal men. J Clin Endocrinol Metab 1992; 74: 75–83.
Amory JK, Anawalt BD, Blaskovich PD, Gilchriest J, Nuwayser ES, Matsumoto AM . Testosterone release from a subcutaneous, biodegradable microcapsule formulation (Viatrel) in hypogonadal men. J Androl 2002; 23: 84–91.
Behre HM, Nieschlag E . Testosterone buciclate (20 Aet-1) in hypogonadal men: pharmacokinetics and pharmacodynamics of the new long-acting androgen ester. J Clin Endocrinol Metab 1992; 75: 1204–1210.
Guay AT, Jacobson J, Perez JB, Hodge MB, Velasquez E . Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res 2003; 15: 156–165.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seftel, A. Testosterone replacement therapy for male hypogonadism: Part III. Pharmacologic and clinical profiles, monitoring, safety issues, and potential future agents. Int J Impot Res 19, 2–24 (2007). https://doi.org/10.1038/sj.ijir.3901366
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901366
Keywords
This article is cited by
-
Cross-sex hormone therapy for gender dysphoria
Journal of Endocrinological Investigation (2015)
-
Aromatase inhibitors in pediatrics
Nature Reviews Endocrinology (2012)
-
Testosterone replacement therapy for late-onset hypogonadism: current trends in Korea
Asian Journal of Andrology (2011)
-
The role of testosterone in erectile dysfunction
Nature Reviews Urology (2010)
-
Ipogonadismo maschile dell’adulto: terapia con testosterone
L'Endocrinologo (2010)