Abstract
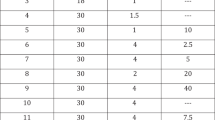
Intracavernous injection of Trimix (Tx) is indicated for patients unsuitable for prostaglandin E1 (PgE1) injection due to lack of response, pain or cost. We believe that the ideal ratio of ingredient doses in Tx is yet to be found. We postulated that increasing the doses of individual drug components in an orderly manner would convey important data on penile hemodynamic response. Such information is needed to choose an effective and less costly alternative to PgE1 with least side effects. We set out to evaluate the impact of varying the ingredient dosage on response and short-term safety of Tx compared with PgE1. We prospectively randomized 180 consecutive patients with erectile dysfunction into nine equal groups and each group received a different dose of Tx, namely phentolamine (1 mg) plus one dose of PgE1 (2.5, 5 or 10 μg) and one dose of papaverine (5, 10 or 20 mg). Each patient was injected with 20 μg PgE1 and one dose of Tx in two clinic visits 1 week apart. Following injection, duplex ultrasound of cavernous arteries and axial rigidometry were carried out. Patients ranked the quality of erection, estimated overall satisfaction and reported time to detumescence and side effects. Patients' mean age was 50.5±11.7 y with underlying organic condition in 91.1%. There were no significant differences between PgE1 and Tx with regard to peak cavernous artery flow, time to erection, patients' satisfaction, average axial rigidity and pain. PgE1 produced higher end diastolic velocity, shorter duration of erection and less priapism. Patients did not show a preference for either drug or any particular dosage. We conclude that even at the smallest dose of ingredients of Tx, there are no significant differences in hemodynamic effects, rigidity, pain and self-satisfaction between the two drugs. However, Tx produces a longer duration of erection and more priapism than PgE1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Shabsigh R et al. Intracavernous alprostadil alfadex (EDEX/VIRIDAL) is effective and safe in patients with erectile dysfunction after failing sildenafil (Viagra). Urology 2000; 55: 477–480.
Hatzichristou DG et al. Sildenafil versus intracavernous injection therapy: efficacy and preference in patients on intracavernous injection for more than 1 year. J Urol 2000; 164: 1197–1200.
Montague DK et al. Clinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunction. The American Urological Association. J Urol 1996; 156: 2007–2011.
von Heyden B et al. A prostaglandin E1 dose–response study in man. J Urol 1993; 150: 1825–1828.
Ismail M, Abbott L, Hirsch IH . Experience with intracavernous PGE-1 in the treatment of erectile dysfunction: dose considerations and efficacy. Int J Impot Res 1997; 9: 39–42.
Bennett AH, Carpenter AJ, Barada JH . An improved vasoactive drug combination for a pharmacological erection program. J Urol 1991; 146: 1564–1565.
Govier FE et al. Experience with triple-drug therapy in a pharmacological erection program. J Urol 1993; 150: 1822–1824.
Marshall GA, Breza J, Lue TF . Improved hemodynamic response after long-term intracavernous injection for impotence. Urology 1994; 43: 844–848.
Shenfeld O et al. Papaverine–phentolamine and prostaglandin E1 versus papaverine–phentolamine alone for intracorporeal injection therapy: a clinical double-blind study. J Urol 1995; 154: 1017–1019.
Mulhall JP et al. The causes of patient dropout from penile self-injection therapy for impotence. J Urol 1999; 162: 1291–1294.
Montorsi F et al. Current status of local penile therapy. Int J Impot Res 2002; 14(Suppl 1): S70–S81.
Israilov S et al. Intracavernous injections for erectile dysfunction in patients with cardiovascular diseases and failure or contraindications for sildenafil citrate. Int J Impot Res 2002; 14: 38–43.
Rosen RC et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999; 11: 319–326.
Kunelius P, Lukkarinen O . Intracavernous self-injection of prostaglandin E1 in the treatment of erectile dysfunction. Int J Impot Res 1999; 11: 21–24.
Hatzichristou D et al. Diagnostic steps in the evaluation of patients with erectile dysfunction. J Urol 2002; 168: 615–620.
Bechara A et al. Prostaglandin E1 versus mixture of prostaglandin E1, papaverine and phentolamine in nonresponders to high papaverine plus phentolamine doses. J Urol 1996; 155: 913–914.
Goldstein I et al. Axial penile rigidity as primary efficacy outcome during multi-institutional in-office dose titration clinical trials with alprostadil alfadex in patients with erectile dysfunction. Alprostadil Alfadex Study Group. Int J Impot Res 2000; 12: 205–211.
Vanderschueren D et al. A study in patients with erectile dysfunction comparing different formulations of prostaglandin E1. Alprostadil Study Group. J Urol 1995; 154: 1744–1747.
Quam JP et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. Am J Roentgenol 1989; 153: 1141–1147.
Porst H . The rationale for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol 1996; 155: 802–815.
Casabe A et al. Drop-out reasons and complications in self-injection therapy with a triple vasoactive drug mixture in sexual erectile dysfunction. Int J Impot Res 1998; 10: 5–9.
Acknowledgements
We express our thanks to Mis Yvonne Lock for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seyam, R., Mohamed, K., Akhras, A. et al. A prospective randomized study to optimize the dosage of trimix ingredients and compare its efficacy and safety with prostaglandin E1. Int J Impot Res 17, 346–353 (2005). https://doi.org/10.1038/sj.ijir.3901313
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901313
Keywords
This article is cited by
-
Non-pharmacological and drug treatment of autonomic dysfunction in multiple system atrophy: current status and future directions
Journal of Neurology (2023)
-
Current Diagnosis and Management of Erectile Dysfunction
Current Sexual Health Reports (2014)
-
Diagnosis of erectile dysfunction
African Journal of Urology (2009)