Abstract
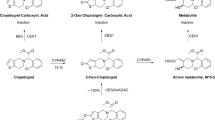
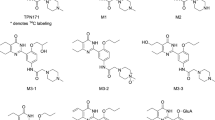
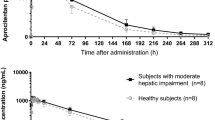
Vardenafil potently inhibits human phosphodiesterase 5 (PDE5) with an IC50 of 0.7 nM. Enhancement of nitric oxide (NO)-induced erections in rabbits by 0.1 mg/kg vardenafil is limited by its pharmacokinetic properties (Tmax=1 h; T1/2=1.2 h), although erectile effects have been observed after 7 h. In humans, vardenafil is rapidly absorbed (Tmax ≈40 min) and more slowly metabolized (T1/2≈4 h), with an absolute bioavailability of 14.5% (vs 40% for sildenafil). Although the consumption of high-fat meals does not affect the drug’s relative bioavailability, it retards intestinal absorption. Coadministration of CYP3A4 inhibitors such as ritonavir can affect hepatic metabolism. M1, an active metabolite of vardenafil, is a four-fold-less potent inhibitor of PDE5 than its parent compound, contributing approximately 7% to vardenafil’s overall efficacy. The side effects of all selective PDE5 inhibitors commonly include vasodilation, small reductions in blood pressure, headache, and nasal congestion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Saenz de Tejada I et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int J Impot Res 2001; 13: 282–290.
Choi S et al. Efficacy of vardenafil and sildenafil in facilitating penile erection in an animal model. J Androl 2002; 23: 332–337.
Bischoff E et al. The oral efficacy of vardenafil hydrochloride for inducing penile erection in a conscious rabbit model. J Urol 2001; 165: 1316–1318.
Giuliano F et al. Proerectile effect of vardenafil: in vitro experiments in rabbits and in vivo comparison with sildenafil in rats. Eur Urol 2003; 44: 731–736.
Klotz T et al. Vardenafil increases penile rigidity and tumescence in erectile dysfunction patients: a RigiScan and pharmacokinetic study. World J Urol 2001; 19: 32–39.
Bischoff E et al. Vardenafil improved erection at four times the plasma half life in a conscious rabbit model. Int J Impot Res 2002; 14(Suppl 3): S42.
Wallis RM, Corbin JD, Francis SH, Ellis P . Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol 1999; 83(5A): 3C–12C.
Rehse K, Scheffler H, Reitner N . Interaction of Viagra with the NO donors molsidomine and RE 2047 with regard to antithrombotic and blood pressure-lowering activities. Arch Pharm (Weinheim) 1999; 332: 182–184.
Berkels R et al. Modulation of human platelet aggregation by the phosphodiesterase type 5-inhibitor sildenafil. J Cardiovasc Pharmacol 2001; 37: 413–421.
Prickaerts J et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 2002; 113: 351–361.
Baratti CM, Boccia MM . Effects of sildenafil on long-term retention of an inhibitory avoidance response in mice. Behav Pharmacol 1999; 10: 731–737.
Schultheiss D et al. Central effects of sildenafil (Viagra) on auditory selective attention and verbal recognition memory in humans: a study with event-related brain potentials. World J Urol 2001; 19: 46–50.
Hoheisel U, Unger T, Mense S . A block of spinal nitric oxide synthesis leads to increased background activity predominantly in nociceptive dorsal horn neurones in the rat. Pain 2000; 88: 249–257.
Asomoza-Espinosa R et al. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol 2001; 418: 195–200.
Dundar M, Kocak I, Dundar SO, Erol H . Evaluation of side effects of sildenafil in a group of young healthy volunteers. Int Urol Nephrol 2001; 32: 705–708.
Fink HA et al. Sildenafil for male erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med 2002; 162: 1349–1360.
Kruuse C, Thomsen LL, Jacobsen TB, Olesen J . The phosphodiesterase 5 inhibitor sildenafil has no effect on cerebral blood flow or blood velocity, but nevertheless induces headache in healthy subjects. J Cereb Blood Flow Metab 2002; 22: 1124–1131.
Zhang R et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke 2002; 33: 2675–2680.
Cellek S, Rees RW, Kalsi J . A Rho-kinase inhibitor, soluble guanylate cyclase activator and NO-releasing PDE5 inhibitor: novel approaches to erectile dysfunction. Expert Opin Investig Drugs 2002; 11: 1563–1573.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bischoff, E. Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events. Int J Impot Res 16 (Suppl 1), S34–S37 (2004). https://doi.org/10.1038/sj.ijir.3901213
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901213
Keywords
This article is cited by
-
Sulfur-containing therapeutics in the treatment of Alzheimer’s disease
Medicinal Chemistry Research (2021)
-
Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans
Psychopharmacology (2015)
-
Simple and sensitive liquid chromatography–tandem mass spectrometry methods for quantification of tadalafil in rat plasma: application to pharmacokinetic study in rats
Archives of Pharmacal Research (2013)
-
Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts
Acta Pharmacologica Sinica (2009)
-
Relaxant effects of an alkaloid-rich fraction from Aspidosperma ulei root bark on isolated rabbit corpus cavernosum
International Journal of Impotence Research (2008)