Abstract
Recent reports have indicated that the neoplastic T cells of angioimmunoblastic T-cell lymphoma express CD10. It has been suggested that the demonstration of a CD10+ T-cell population may assist in establishing a diagnosis of angioimmunoblastic T-cell lymphoma and in distinguishing angioimmunoblastic T-cell lymphoma from other peripheral T-cell lymphomas. It has been unclear, however, whether this phenotypically unusual T-cell population might be present in other settings as well. In this report, we have retrospectively examined 64 cases of lymph node and solid tissue biopsies for the presence of CD10+ T cells using multicolor flow cytometry. Discrete populations of CD10+ T cells were found in 5 of 28 cases (18%) of reactive lymphoid hyperplasia, 4 of 17 cases (23%) of follicular lymphoma, and 9 of 19 cases (47%) of marginal zone B-cell lymphomas. The CD10+ T cells constituted 1–6% of total cells analyzed and ≤14% of the total T-cell population. Using two-color immunohistochemical stains, many of the CD10+ PAX5-negative presumptive T cells were found to be located within germinal centers. These findings indicate that a normal small subset of CD10+ peripheral T cells exists and, at least when present in small numbers, should not be considered an indication of a T-cell neoplasm.
Similar content being viewed by others
INTRODUCTION
The expression of CD10 by lymphoid cells correlates with specific stages of development and maturation (1, 2). Lymphoblasts, of both B- and T-cell lineages, demonstrate expression of CD10 that is lost upon progression to a mature phenotype. Mature B cells express CD10 again during the germinal center reaction, with subsequent loss of expression as the B cells leave the germinal centers as memory B cells and/or plasma cells (1, 3). The expression of CD10 by mature T cells, however, has not been well characterized.
Angioimmunoblastic T-cell lymphoma is a rare malignant neoplasm of mature T cells associated with a spectrum of morphologic features that in some cases may overlap with the findings in abnormal reactive proliferations (4, 5). Definitive diagnosis often can be difficult without evidence of clonality as demonstrated by either genotypic or cytogenetic studies. There is therefore great interest in identifying potential immunophenotypic findings that could aid in the identification of angioimmunoblastic T-cell lymphoma. Recent reports have indicated that the neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10 and have suggested that the demonstration of a CD10+ T-cell population may aid in the diagnosis of this specific type of T-cell lymphoma (6, 7, 8). However, it has been unclear whether CD10+ mature T cells may also be present in other lymphoid proliferations as well and whether CD10 expression by mature T cells represents a normal T-cell subset or an aberrant phenotype.
In this study, we analyzed flow cytometry data and histograms from 64 cases of lymph node or solid tissue biopsies containing reactive lymphoid hyperplasia, follicular lymphoma, or marginal zone B-cell lymphoma. The percentage and distribution of CD5+/CD10+/CD19− cells was evaluated, and the T-cell origin of these cells was directly confirmed in four cases using analysis of CD10 and CD3 coexpression. Selected cases were also examined by dual-color immunohistochemical stains using CD10 and the PAX5 nuclear B-cell marker to identify the anatomic location of the CD10+ PAX5− presumptive T cells.
MATERIALS AND METHODS
Case Selection
Cases selected for retrospective analysis included 28 cases of reactive lymphoid hyperplasia, 17 cases of follicular lymphoma, and 19 cases of marginal zone lymphoma. The cases of reactive lymphoid hyperplasia and follicular lymphoma represent cases with diagnostic flow cytometry over a 6-month period at UPMC-Presbyterian and were described in a previous study (9). The cases of marginal zone lymphoma represent all cases of marginal zone lymphoma with diagnostic flow cytometric immunophenotypic studies performed at UPMC-Presbyterian over a 1-year period. In addition, four cases of lymph node biopsies containing CD10+ T cells (3 follicular lymphomas, 1 marginal zone lymphoma) were identified in a prospective fashion during routine practice. Cases of follicular lymphoma and marginal zone lymphoma were chosen for study in part because CD10+ T cells were first noted in such cases by flow cytometric analyses in our routine practice. Furthermore, these cases often contain follicular structures, and the malignant CD10+ T cells of angioimmunoblastic T-cell lymphoma are thought to have a relationship to abnormal follicular structures (6, 7, 8). All diagnoses were rendered according to WHO criteria using histologic, immunohistochemical, and/or flow cytometric findings (10).
Flow Cytometry
Flow cytometric analysis was performed as previously described (11). The presence of CD10+ T-cell populations was evaluated via three-color analysis using CD5-FITC (Clone L17F12, Becton Dickinson, Franklin Lakes, NJ), CD10-PE (Clone SS2/36, DAKO, Carpinteria, CA), and CD19-PC5 (Clone J4.119, Beckman Coulter, Fullerton, CA). Data were acquired on 10,000 cells per sample using a Becton Dickinson FACScalibur with CellQuest software (Becton Dickinson). Data were analyzed using CellQuest and Paint-a-Gate software (Becton Dickinson). CD10+ T cells were identified and quantified by gating first on CD10-positive events with subsequent gating on CD5+CD19− events. In the prospectively identified cases, the cell suspensions were also analyzed in similar fashion after staining with one of the following antibody combinations: CD10-PE/CD3-FITC (Clone SK7, Becton Dickinson), CD10-PE/CD3-FITC/CD19-PC5, CD10-PE/CD3-PerCP (Clone SK7, Becton Dickinson), or CD10-PE/CD3-PerCP/CD57-FITC (Clone HNK-1, Becton Dickinson).
Immunohistochemistry
Two-color immunohistochemical stains were performed on formalin-fixed, paraffin-embedded tissues as previously described (12). Briefly, slides were deparaffinized, pretreated by microwaving in citrate buffer (10 mm citrate, pH 6.0), and manually stained using the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA). Slides were stained first with CD10 (DAKO, Carpinteria, CA; 1:10 dilution) and nickel 3,3′-diaminobenzidine chromogen (Vector Laboratories), followed by PAX5 (Transduction Laboratories, Lexington, KY; 1:50 dilution) and Vector Red chromogen (Vector Laboratories). No counterstain was employed.
Statistical Analyses
Statistical analyses were performed as indicated using GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
Routine flow cytometric histograms of lymph node or solid tissue biopsies containing reactive lymphoid hyperplasia, follicular lymphoma, or marginal zone lymphoma were retrospectively reviewed for the presence of CD10+ T cells, identified as cells with a CD5+CD10+CD19− phenotype (Fig. 1). Discrete populations of CD10+ T cells were identified in 5 of 28 cases of reactive lymphoid hyperplasia (18%), 4 of 17 cases of follicular lymphoma (23%), and 9 of 19 cases of marginal zone lymphoma (47%; Table 1). The cases classified as lacking discrete populations of CD10+ T cells displayed few, scattered events in the CD5+CD10+CD19− region that did not form distinct populations in flow cytometric dot plots and represented ≤2% of total events analyzed. The viability of the specimens at the time of analysis in cases with (92 ± 5%) and without (84 ± 18%) discrete CD10+ T-cell populations were similar (P = .07, unpaired Student's t test).
The CD10+ T cells consistently demonstrated strong expression of CD5, similar to that of other T cells, and tended to show limited variability in forward- and side-scatter characteristics (Fig. 1). The proportion of CD10+ T cells ranged from 1 to 6% of total events analyzed, and accounted for ≤14.4% of all T cells present in the specimen. In cases of marginal zone lymphoma, the CD10+ T cells represented a larger proportion of the CD10+ lymphoid population (≤75.5% of CD10+ cells) than in reactive lymphoid hyperplasia or follicular lymphoma.
In addition to the retrospective analysis, four B-cell lymphomas containing CD10+ T cells with sufficient material for further flow cytometric studies were identified in a prospective fashion (three follicular lymphomas and one marginal zone lymphoma). In each of these cases, definitive coexpression of CD3 and CD10 was identified (Fig. 2). Similar percentages of CD10+ T cells were enumerated whether identified as CD3+CD10+ cells or as CD5+CD10+CD19− cells (Table 2), indicating that at least the vast majority of CD10+ T cells also express CD3 and that either antibody combination provides an accurate determination of the number of CD10+ T cells present. The intensity of CD3 expression tended to be slightly lower in the CD10+ T cells (32.1 ± 5.9, MFI ± SEM) than in the CD10− T cells (41.4 ± 8.1, MFI ± SEM), although this difference was not statistically significant (P = .138, paired t test). In the one case in which CD57 expression was analyzed, the majority of CD10+ T cells lacked CD57 expression (data not shown).
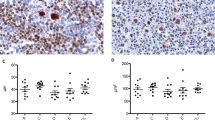
To identify the anatomic location of the CD10+ T cells, two-color immunohistochemical stains were performed using antibodies against CD10 and the nuclear pan–B-cell marker, PAX5, on all cases with >2% CD10+ T cells and available paraffin blocks (two reactive lymphoid hyperplasias, two follicular lymphomas, and four marginal zone lymphomas). Sections of benign tonsils were also stained in similar fashion. Interpretation of these stains was complicated as the somewhat diffuse-appearing CD10 staining of germinal center B cells frequently appeared to be partially masked by PAX5 staining that often gave increased cytoplasmic background staining in germinal centers. However, the stains did clearly identify PAX5-negative lymphoid cells with strong membrane positivity for CD10, consistent with CD10+ T cells. In cases of reactive lymphoid hyperplasia and in benign control tonsillar tissues, the CD10+ T cells appeared to be distributed as single cells within follicles and occasionally in mantle zones, with rare scattered cells in the interfollicular areas (Fig. 3, A–B). Marginal zone lymphoma cases with residual germinal centers also contained CD10+ T cells primarily within follicular structures (Fig. 3, C–D). In two marginal zone lymphoma cases, residual germinal centers were not present in the sections analyzed, and CD10+PAX5− cells were present scattered throughout the lymphomatous infiltrate. Two-color immunostains performed on cases of FL could not be satisfactorily interpreted because of the density of CD10+/PAX5+ cells present.
Anatomic localization of CD10+ presumptive T cells. Two-color immunohistochemical stains for CD10 (black) and PAX5 (red) demonstrate CD10+PAX5− cells primarily within germinal centers in benign control tonsils (A, B). A case of marginal zone B-cell lymphoma contains CD10+PAX5− cells within residual follicular structures (C). Note the presence of few CD10+PAX5+ germinal center B cells and more numerous CD10+PAX5− T cells at higher magnification (D). Magnification: 100× (A, C) and 400× (B, D).
DISCUSSION
The CD10 antigen is a 100-kDa cell surface protein with endopeptidase activity (1, 2) that, although originally described as a potential leukemia-specific antigen (13), is now known to be expressed by a variety of hematopoietic, epithelial, and mesenchymal tissues (14, 15, 16, 17). Among hematopoietic cells, CD10 expression is found in granulocytes and in B and T lymphocytes at particular stages of maturation. CD10 expression within the B-cell lineage has been very well characterized. Benign CD10+ B cells include B lymphoblasts as well as mature germinal center B cells (1, 18). CD10 is also characteristically expressed by several B-cell malignancies, including a subset of lymphoblastic lymphomas and leukemias, and mature B-cell lymphomas of follicular center cell origin including follicular lymphomas and a subset of diffuse large B-cell lymphomas (1, 3, 11, 12, 18). The evaluation of CD10 expression has therefore become an important part of the routine evaluation of B-cell neoplasms.
In contrast, the expression of CD10 by T lymphocytes has received less attention in the literature. CD10 is expressed by a subset of immature thymocytes and is generally thought to be absent on mature T cells (1, 2). Cutrona and colleagues (19), however, reported that T cells subjected to apoptotic stimuli in vitro express CD10 and that apoptotic T cells isolated from HIV-infected lymph nodes are also CD10+. More recently, CD10+ T cells have also been described in two forms of mature T-cell lymphomas. Attygalle et al. (6) demonstrated that CD10+ T cells could be identified by paraffin section immunohistochemistry in 90% of cases of angioimmunoblastic T-cell lymphoma, where they tended to be concentrated near the periphery of germinal centers in cases with residual follicular structures. The CD10+ T cells always represented a minority of the CD3+ T cells present, ranging from 5–30% of all T cells. Microdissection studies indicated that the CD10+ cells had clonal T-cell receptor rearrangements, whereas the CD10− T cells were polyclonal. CD10+ T cells were reportedly absent by paraffin section immunohistochemistry in 10 cases of other peripheral T-cell lymphomas and in 10 reactive lymph nodes. Similarly, Yuan and colleagues (8) have reported in abstract form that two of three cases of angioimmunoblastic T-cell lymphoma contained CD10+ T cells, whereas two other cases of peripheral T-cell lymphoma lacked detectable CD10+ T cells. Both groups of investigators reported that the neoplastic CD10+ T cells express CD4 and, at least in a subset of the neoplastic cells, BCL-6. Other investigators have reported CD10+ T cells in two of three cases reported as follicular T-cell lymphoma (20). The relationship of these latter cases to angioimmunoblastic T-cell lymphoma is currently unclear. These findings suggest that the demonstration of CD10 expression by neoplastic T cells may aid in establishing a diagnosis of specific forms of T-cell lymphoma.
To search for a possible benign counterpart to the neoplastic T cells of angioimmunoblastic T-cell lymphoma, and to further evaluate the diagnostic significance of identifying populations of CD10+ T cells, we retrospectively examined flow cytometric data and histograms from 64 lymph node or other solid tissue biopsies. We demonstrated discrete populations of CD10+ T cells in 5 of 28 cases of reactive lymphoid hyperplasia (18%), 4 of 17 cases of follicular lymphoma (23%), and 9 of 19 cases of marginal zone lymphoma (47%). In four additional prospectively identified cases, definitive coexpression of CD3 and CD10 was identified. The frequency of these cells in other forms of malignant lymphoma not examined in this study remains to be determined. It is unclear whether, in cases lacking distinct populations of CD10+ T cells, such cells are completely absent or are simply present at a level below the limits of detection with routine flow cytometric analysis. We could not identify specific morphologic or other phenotypic differences between the cases with and without distinct CD10+ T-cell populations.
Two-color paraffin section immunohistochemistry was then used to identify the location of the CD10+ T cells in select cases. In the absence of a good nuclear T-cell marker, and with the known difficulties in interpreting paraffin section double-surface immunostains, especially when the cells of interest are infrequent and when negatively staining cells could easily be obscured by numerous surrounding positive cells, a strategy was employed using a nuclear B-cell–specific marker (PAX5) and a CD10 surface stain. The CD10+ T cells identified by flow cytometry would therefore be seen in the tissue sections as CD10+ PAX5− mononuclear cells. Cases of reactive lymphoid hyperplasia contained CD10+PAX5− presumptive T cells primarily within follicles with scattered cells in the interfollicular areas. The possibility that some of the cells were non–T cells could not be ruled out; however, virtually all B cells are PAX5+, the cells did not resemble neutrophils, and the prominent intrafollicular distribution did not suggest a significant NK cell population. As also described by others using paraffin section immunohistochemistry (6), the CD10 staining on T cells had a more intense, crisp membrane pattern that differed from the less intense, more diffuse membrane and cytoplasmic staining seen in germinal center B cells. The finding of a predominantly intrafollicular location raises the possibility that these cells may have a relationship to the neoplastic cells of angioimmunoblastic T-cell lymphoma or the so-called follicular T-cell lymphoma, as in these settings the malignant CD10+ T cells appear to have some association with follicular structures. From these stains, CD10 would appear to be present on only a minority of intrafollicular T cells. The relationship of these CD10+ T cells to other intrafollicular T-cell subsets, such as those expressing CD57 or BCL-6, is unclear and merits further investigation.
Interestingly, CD10+ T-cell populations were present in a greater proportion of marginal zone lymphoma cases than in cases of reactive lymphoid hyperplasia or follicular lymphoma. In marginal zone lymphoma cases, the CD10+ T cells accounted for ≤75.5% of total CD10+ cells, possibly due to the presence of follicular colonization and associated loss of benign CD10+ B-cells. The CD10+ T cells in marginal zone lymphoma cases, as with cases of reactive lymphoid hyperplasia and follicular lymphoma, made up only a minority of total T cells present (range: 4.2–14.4%). Two color immunostains demonstrated that the CD10+ presumptive T cells in these cases were also located primarily within follicular structures in the cases where residual follicles were present.
The physiologic role of CD10 expression by T cells, if any, is currently unknown. Cutrona et al. (19) reported that apoptotic T cells were CD10+ and showed decreased CD3 expression. The cases in the current study also tended to show somewhat lower CD3 expression in the four cases in which such analysis could be performed, although this decreased intensity was not statistically significant. The previous report found CD10+ T cells in HIV-positive lymph nodes to be present at much greater frequency (≤70% of all T cells) than were identified in the current study. It is unclear whether the large percentage of CD10+ T cells observed by these investigators is related primarily to the relative rates of apoptosis, or alternatively, whether a selective expansion of CD10+ T cells may occur in HIV-infected lymph nodes. Additional studies will be required to determine whether CD10+ T cells represent only T cells undergoing cell death or a stable T-cell subset.
This study demonstrates that benign CD10+ T cells may be found by flow cytometric studies in a variety of settings other than angioimmunoblastic T-cell lymphoma. The lack of CD10+ T cells in cases of reactive lymphoid hyperplasia reported by others using paraffin section immunohistochemistry (6) likely reflects the superiority of flow cytometry in identifying small, phenotypically distinct cell populations. Although the CD10+ T cells identified in the current study made up only a small proportion of total cells (1–6% of cells analyzed), this percentage is similar to that found in many cases of angioimmunoblastic T-cell lymphoma (6). Therefore, although the presence of a CD10+ T-cell population may be characteristic of angioimmunoblastic T-cell lymphoma, establishing a definitive diagnosis requires correlation with all available morphologic and immunophenotypic findings.
Note Added in Proof
Study of three additional cases shows >90% of the CD10+ T-cells are CD4+ but <50% are CD57+.
References
Arber D . CD10: a review. Appl Immunohistochem Mol Morphol 1997; 5: 125–40.
LeBien TW, McCormack RT . The common acute lymphoblastic leukemia antigen (CD10)—emancipation from a functional enigma. Blood 1989; 73: 625–35.
Almasri NM, Iturraspe JA, Braylan RC . CD10 expression in follicular lymphoma and large cell lymphoma is different from that of reactive lymph node follicles. Arch Pathol Lab Med 1998; 122: 539–44.
Jaffe E, Ralfkiaer E . Angioimmunoblastic T-cell lymphoma. In: Jaffe E, Harris N, Stein H, Vardiman J, editors. Tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001. p. 225–6.
Ferry JA . Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol 2002; 9: 273–9.
Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood 2002; 99: 627–33.
Attygalle A, Diss TC, Isaacson PG, Dogan A . CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma [abstract]. Mod Pathol 2002: 964.
Yuan C, Vergilio J, Harris N, Bagg A . Angioimmunoblastic T-cell lymphoma. A neoplasm of intrafollicular CD10+, BCL-6+, CD4+ memory T cells [abstract]? Mod Pathol 2002: 1125.
Cook J, Craig F, Swerdlow SH . Bcl-2 expression by multicolor flow cytometric analysis assists in the diagnosis of follicular lymphoma in lymph node and bone marrow. Am J Clin Pathol 2003; 119: 145–51.
Jaffe E, Harris N, Stein H, Vardiman J . Tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2001.
Chen CC, Raikow RB, Sonmez-Alpan E, Swerdlow SH . Classification of small B-cell lymphoid neoplasms using a paraffin section immunohistochemical panel. Appl Immunohistochem Mol Morphol 2000; 8: 1–11.
King BE, Chen C, Locker J, Kant J, Okuyama K, Falini B, et al. Immunophenotypic and genotypic markers of follicular center cell neoplasia in diffuse large B-cell lymphomas. Mod Pathol 2000; 13: 1219–31.
Brown G, Hogg N, Greaves M . Candidate leukaemia-specific antigen in man. Nature 1975; 258: 454–6.
Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G . Expression of the common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol 1985; 38: 1002–6.
Metzgar RS, Borowitz MJ, Jones NH, Dowell BL . Distribution of common acute lymphoblastic leukemia antigen in nonhematopoietic tissues. J Exp Med 1981; 154: 1249–54.
Braun MP, Martin PJ, Ledbetter JA, Hansen JA . Granulocytes and cultured human fibroblasts express common acute lymphoblastic leukemia-associated antigens. Blood 1983; 61: 718–25.
Yaziji H, Gown AM . Immunohistochemical analysis of gynecologic tumors. Int J Gynecol Pathol 2001; 20: 64–78.
Dogan A, Bagdi E, Munson P, Isaacson PG . CD10 and BCL-6 expression in paraffin sections of normal lymphoid tissue and B-cell lymphomas. Am J Surg Pathol 2000; 24: 846–52.
Cutrona G, Leanza N, Ulivi M, Melioli G, Burgio VL, Mazzarello G, et al. Expression of CD10 by human T cells that undergo apoptosis both in vitro and in vivo. Blood 1999; 94: 3067–76.
de Leval L, Savilo E, Longtine J, Ferry JA, Harris NL . Peripheral T-cell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am J Surg Pathol 2001; 25: 395–400.
Acknowledgements
We are grateful to Kim Stanis-Fuhrer for expert assistance with two-color immunohistochemical stains and to the UPMC-Presbyterian flow cytometry laboratory for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cook, J., Craig, F. & Swerdlow, S. Benign CD10-Positive T Cells in Reactive Lymphoid Proliferations and B-Cell Lymphomas. Mod Pathol 16, 879–885 (2003). https://doi.org/10.1097/01.MP.0000084630.64243.D1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000084630.64243.D1
Keywords
This article is cited by
-
Spatial profiling of chromatin accessibility in mouse and human tissues
Nature (2022)
-
Peripheral T-cell lymphomas of follicular helper T-cell type frequently display an aberrant CD3−/dimCD4+ population by flow cytometry: an important clue to the diagnosis of a Hodgkin lymphoma mimic
Modern Pathology (2016)
-
Atypical angioimmunoblastic T-cell lymphomas masquerading as systemic polyclonal B-immunoblastic proliferation
Virchows Archiv (2012)
-
Identification of circulating CD10 positive T cells in angioimmunoblastic T-cell lymphoma
Leukemia (2006)